15.4 The Oxidation of Carbon Fuels Is an Important Source of Cellular Energy
✓ 5 Describe the relation between the oxidation state of a carbon molecule and its usefulness as a fuel.
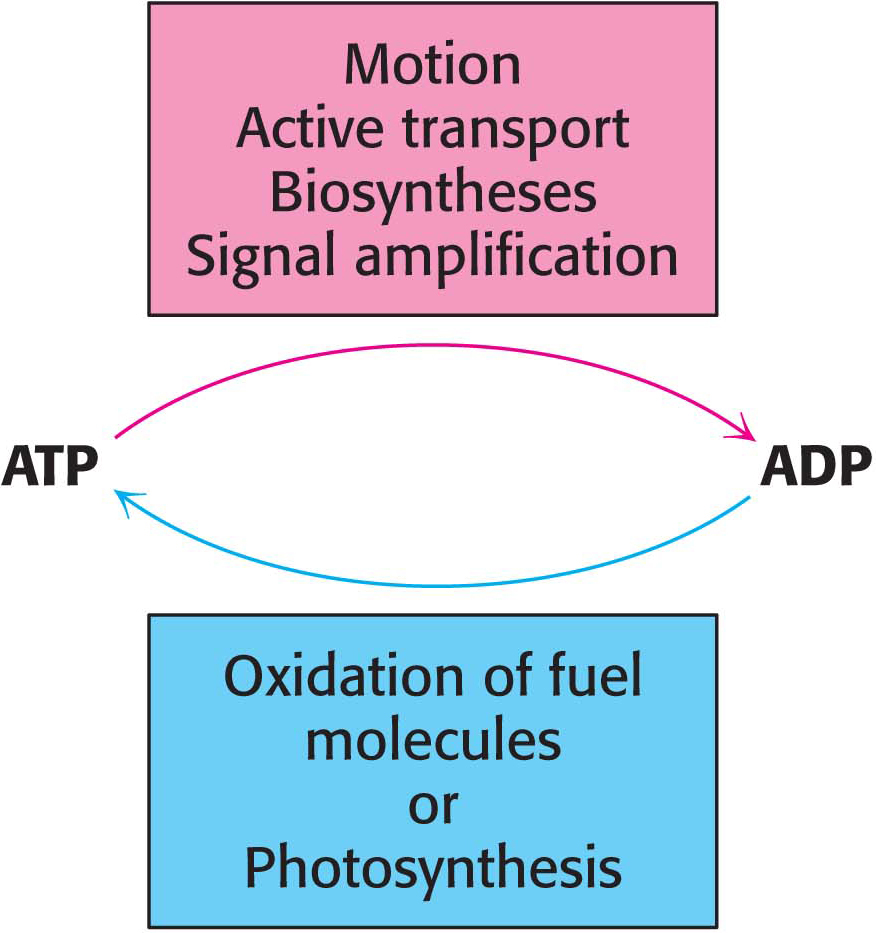
ATP serves as the principal immediate donor of free energy in biological systems rather than as a long-term storage form of free energy. In a typical cell, an ATP molecule is consumed within a minute of its formation. Although the total quantity of ATP in the body is limited to approximately 100 g, the turnover of this small quantity of ATP is very high. For example, a resting human being consumes about 40 kg (88 pounds) of ATP in 24 hours. During strenuous exertion, the rate of utilization of ATP may be as high as 0.5 kg (1.1 pounds) per minute. For a 2-hour run, 60 kg (132 pounds) of ATP is utilized. Clearly, having mechanisms for regenerating ATP is vital. Motion, active transport, signal amplification, and biosynthesis can take place only if ATP is continually regenerated from ADP (Figure 15.10). The generation of ATP is one of the primary roles of catabolism.
Carbon Oxidation Is Paired with a Reduction
The carbon in fuel molecules—such as glucose and fats—is oxidized to CO2, and the energy released is used to regenerate ATP from ADP and Pi. As discussed earlier, the set of reactions that accomplish transformation are called catabolic reactions or, simply, catabolism. Understanding the transition from the energy inherent in reduced organic molecules—biological fuels—to ATP is a central theme in the study of biochemistry. Let’s examine some of basic principles of carbon oxidation. We will return to this subject many times, most notably in Sections 8 through 10.
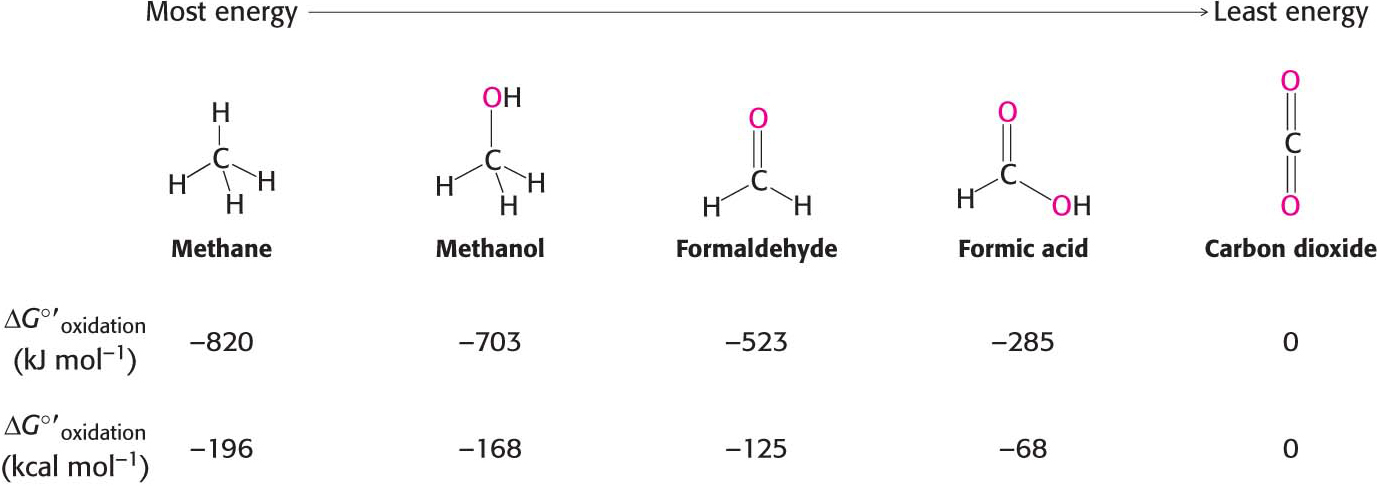
All oxidation reactions include the loss of electrons from the molecule being oxidized—carbon fuels in this case—and the gaining of those electrons by some other molecule, a process termed reduction. Such coupled reactions are called oxidation–reduction or redox reactions. In aerobic organisms, the ultimate electron acceptor in the oxidation of carbon is O2 and the oxidation product is CO2. Consequently, the more reduced a carbon is to begin with, the more free energy is released by its oxidation. Figure 15.11 shows the ΔG°′ of oxidation for one-carbon compounds.
Page 267
Although fuel molecules are more complex (Figure 15.12) than the single-carbon compounds depicted in Figure 15.11, when a fuel is oxidized, the oxidation takes place one carbon atom at a time. The carbon-oxidation energy is used in some cases to create a compound with high phosphoryl-transfer potential and in other cases to create an ion gradient (Section 9). In either case, the end point is the formation of ATP.
Compounds with High Phosphoryl-Transfer Potential Can Couple Carbon Oxidation to ATP Synthesis
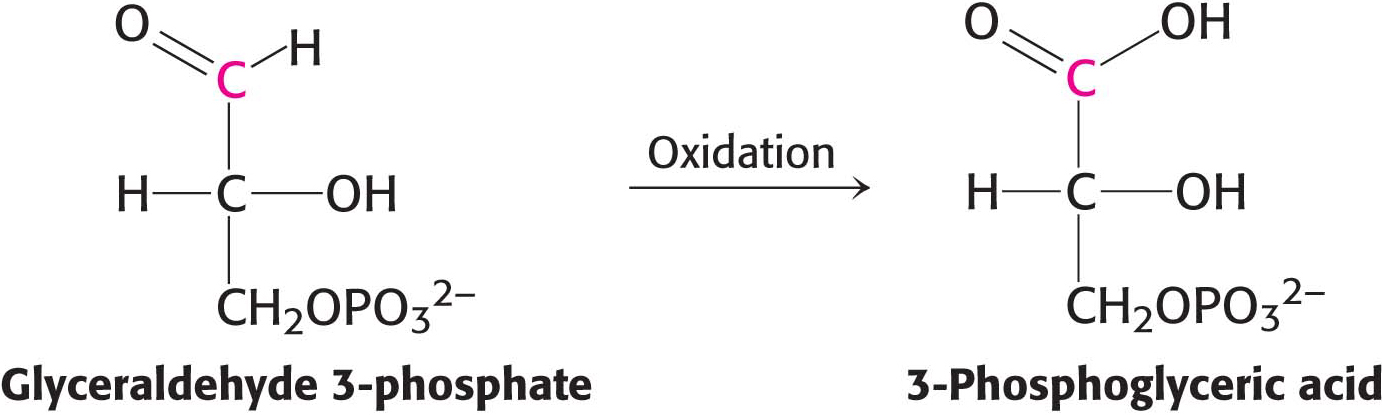
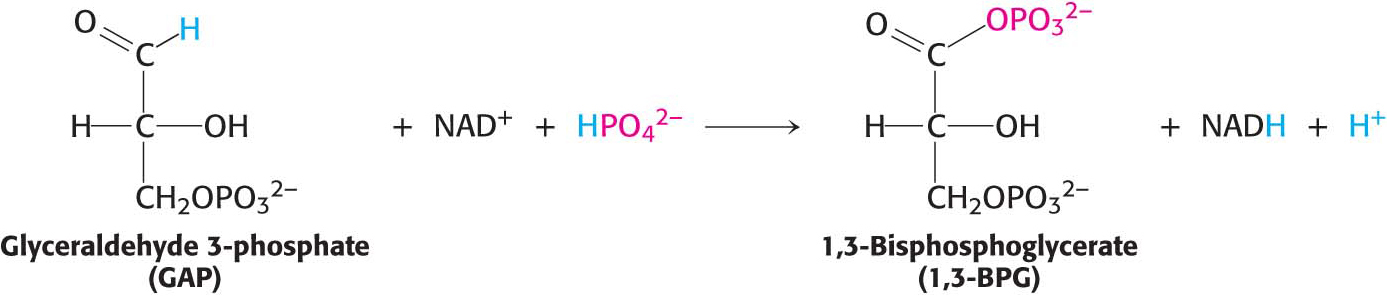
How is the energy released in the oxidation of a carbon compound converted into ATP? As an example, consider glyceraldehyde 3-phosphate, which is a metabolite of glucose formed in the oxidation of that sugar (Chapter 16). The C-1 carbon atom (shown in red) is at the aldehyde-oxidation level and is capable of further oxidation (Figure 15.11). Oxidation of the aldehyde to an acid will release energy.
However, the oxidation does not take place directly. Instead, the carbon oxidation generates an acyl phosphate, 1,3-bisphosphoglycerate. The electrons released are captured by NAD+, which we will consider shortly.
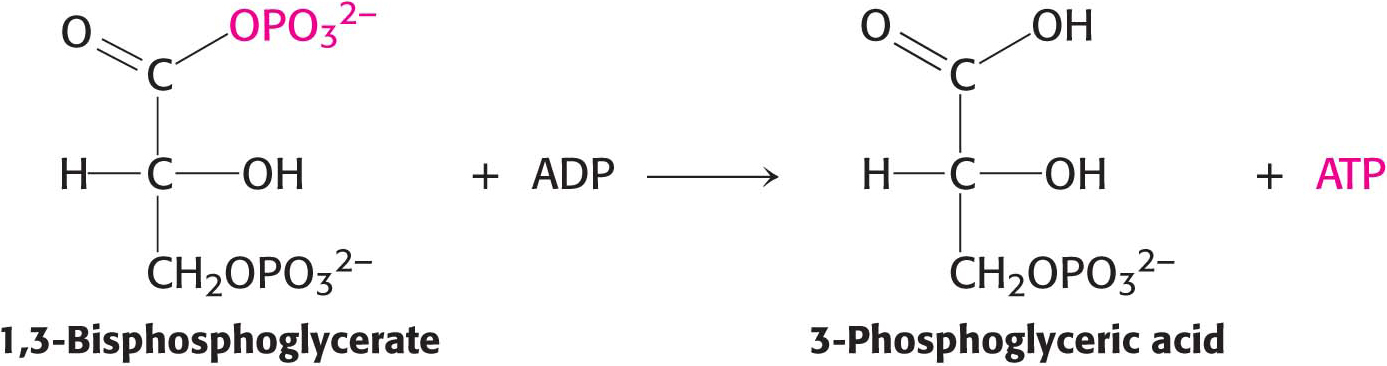
For reasons similar to those discussed for ATP, 1,3-bisphosphoglycerate has a high phosphoryl-transfer potential. Thus, the cleavage of 1,3-BPG can be coupled to the synthesis of ATP.
Page 268
The energy of oxidation is initially trapped as a high-phosphoryl-transfer-potential compound and then used to form ATP. The oxidation energy of a carbon atom is transformed into phosphoryl-transfer potential—first, as 1,3-bisphosphoglycerate and, ultimately, as ATP. We will consider these reactions in mechanistic detail in Chapter 16.