15.5 Metabolic Pathways Contain Many Recurring Motifs
At first glance, metabolism seems intimidating because of the sheer number of reactants and reactions. Nevertheless, there are unifying themes that make comprehending it more manageable. These themes include common metabolites and regulatory schemes that stem from a common evolutionary heritage.
Activated Carriers Exemplify the Modular Design and Economy of Metabolism
We have seen that phosphoryl transfer can be used to drive otherwise thermodynamically unfavorable reactions, alter the energy or conformation of a protein, or serve as a signal to alter the activity of a protein. The phosphoryl-
Activated Carriers of Electrons for Fuel Oxidation. In aerobic organisms, the ultimate electron acceptor in the oxidation of fuel molecules is O2. However, electrons are not transferred directly to O2. Instead, fuel molecules reduce or transfer electrons to special carriers, which are either pyridine nucleotides or flavins. The reduced forms of these carriers then transfer their high-
potential electrons to O2. In other words, these carriers have a higher affinity for electrons than do carbon fuels but a lower affinity for electrons than does O2; consequently, the electrons flow from an unstable configuration (low affinity) to a stable one (high affinity). Indeed, the tendency of these electrons to flow toward a more stable arrangement accounts for their description as being activated. The energy that is released is converted into ATP by mechanisms to be discussed in Chapter 21.
Nicotinamide adenine dinucleotide (NAD+), a pyridine nucleotide, is a major electron carrier in the oxidation of fuel molecules (Figure 15.13). The reactive part of NAD+ is its nicotinamide ring, a pyridine derivative synthesized from the vitamin niacin. In the oxidation of a substrate, the nicotinamide ring of NAD+ accepts a hydrogen ion and two electrons, which are equivalent to a hydride ion (H:−). The reduced form of this carrier is NADH. In the oxidized form, the nitrogen atom carries a positive charge, as indicated by NAD+. Nicotinamide adenine dinucleotide, or NAD+, is the electron acceptor in many reactions of the following type:
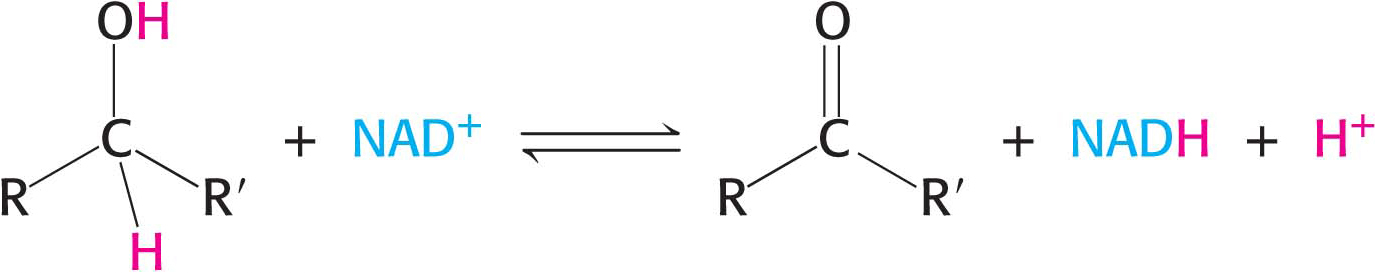
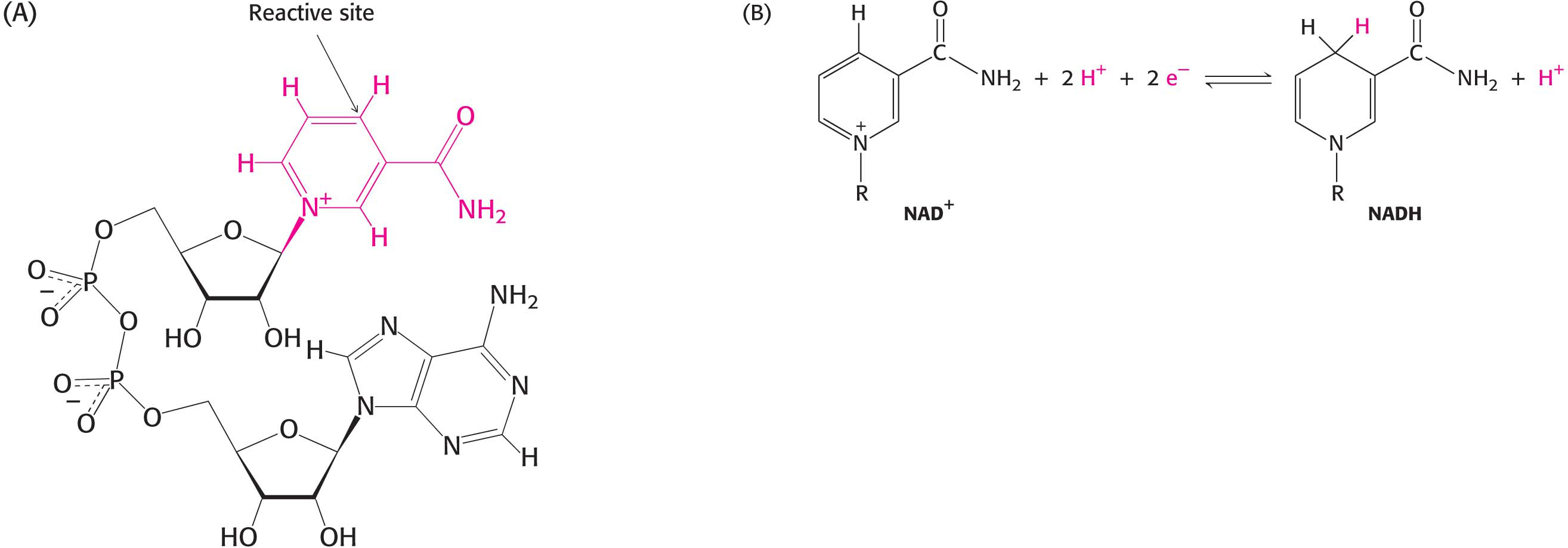
This redox reaction is often referred to as a dehydrogenation because protons accompany the electrons. One proton and two electrons of the substrate are directly transferred to NAD+, whereas the other proton in the solvent as a proton.
The other major electron carrier in the oxidation of fuel molecules is the coenzyme flavin adenine dinucleotide (Figure 15.14). The abbreviations for the oxidized and reduced forms of this carrier are FAD and FADH2, respectively. FAD is the electron acceptor in reactions of the following type:
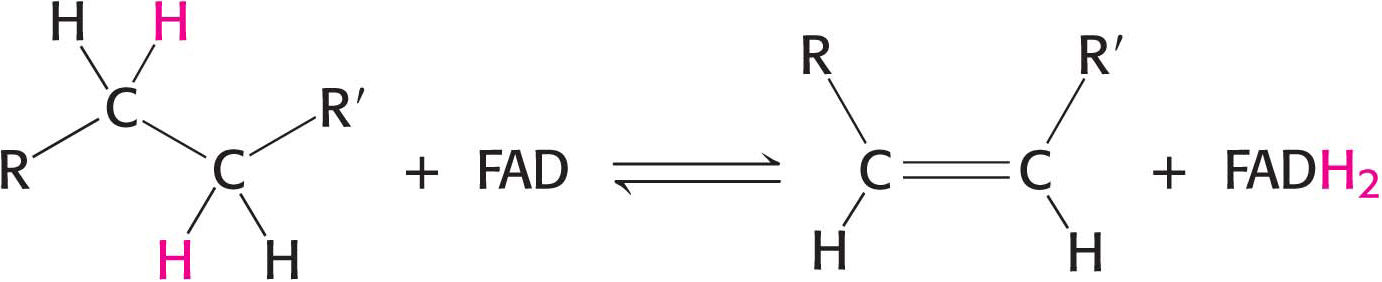
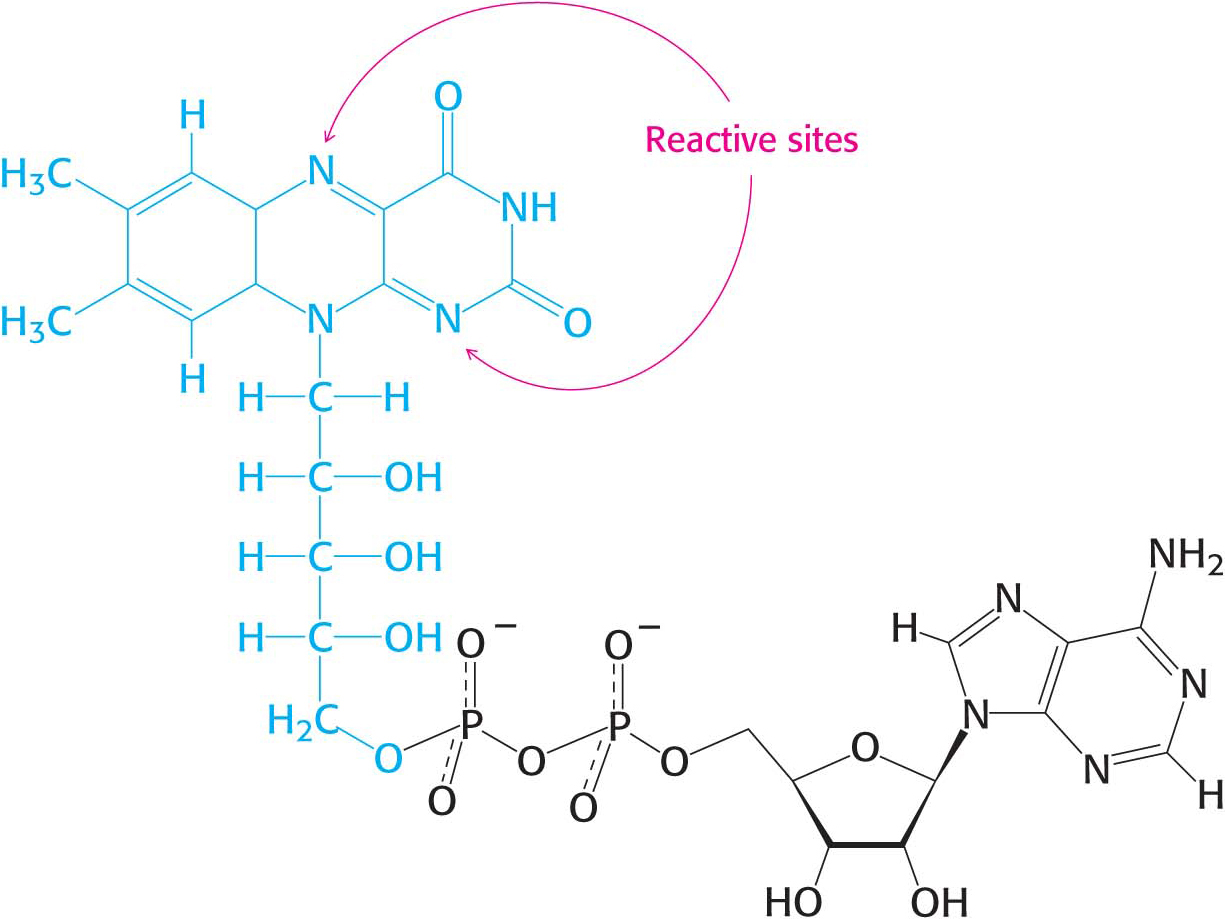
The reactive part of FAD is its isoalloxazine ring, a derivative of the vitamin riboflavin (Figure 15.15). FAD, like NAD+, can accept two electrons. In doing so, FAD, unlike NAD+, takes up two protons. These carriers of high-
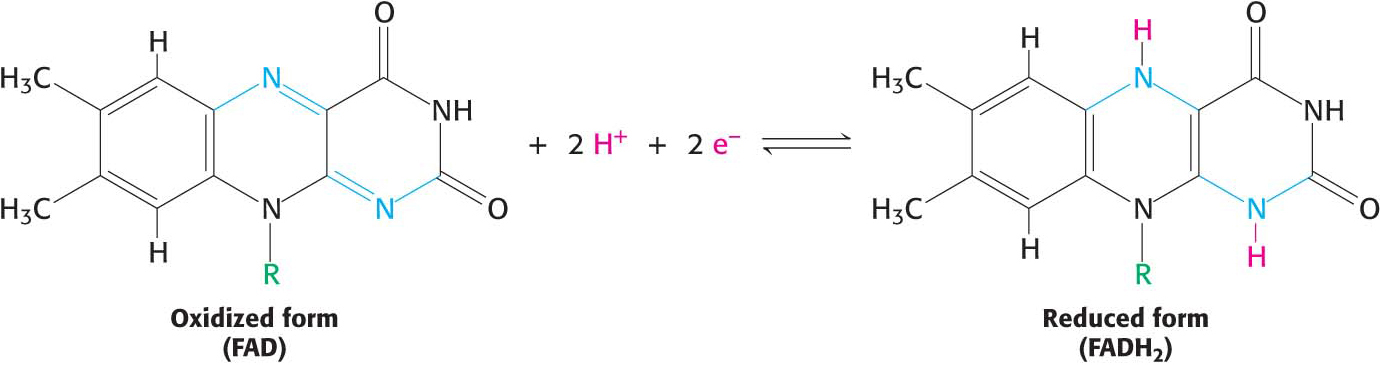
Activated Carriers of Electrons for the Synthesis of Biomolecules. High-
potential electrons are required for anabolic reactions. In most biosyntheses, the precursors are more oxidized than the products and, hence, reducing power is needed in addition to ATP. This process is called reductive biosynthesis. For example, in the biosynthesis of fatty acids, the keto group of a two- carbon unit is reduced to a methylene group in several steps. This sequence of reactions requires an input of four electrons: 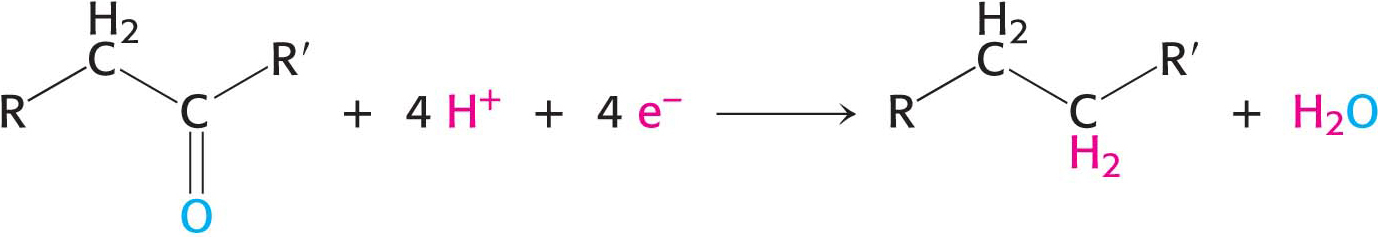
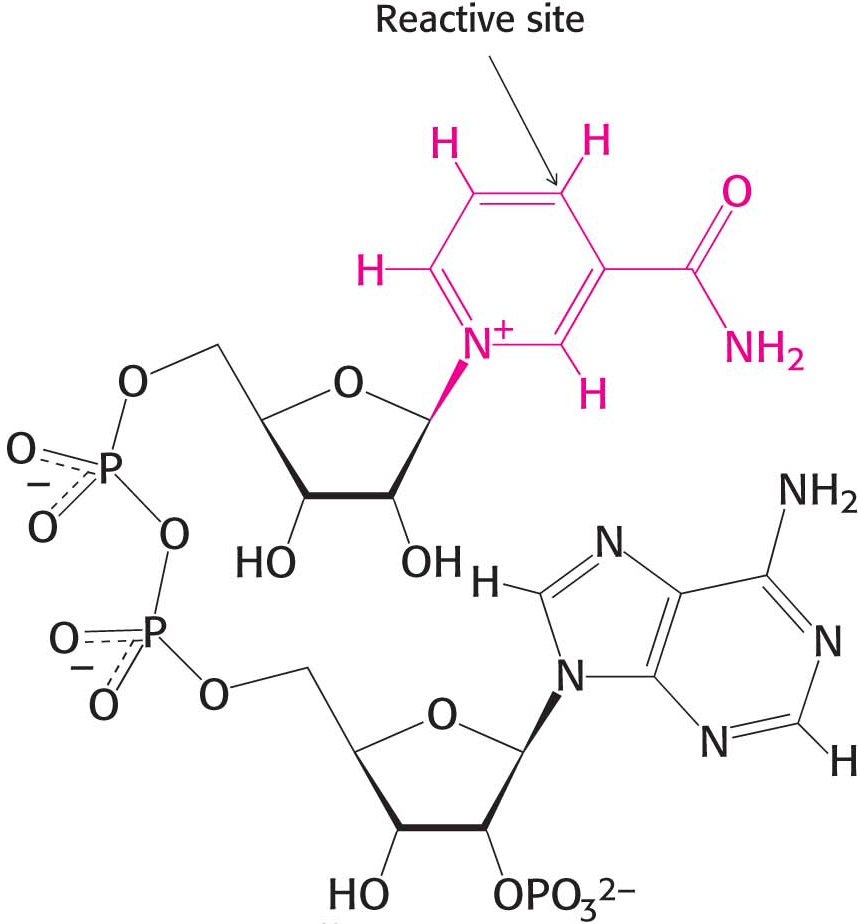
The electron donor in most reductive biosyntheses is NADPH, the reduced form of nicotinamide adenine dinucleotide phosphate (NADP+). NADPH differs from NADH in that the 2′-hydroxyl group of its adenosine moiety is esterified with phosphate (Figure 15.16). NADPH carries electrons in the same way as NADH. However, NADPH is used almost exclusively for reductive biosyntheses, whereas NADH is used primarily for the generation of ATP. The extra phosphoryl group on NADPH is a tag that enables enzymes to distinguish between high-
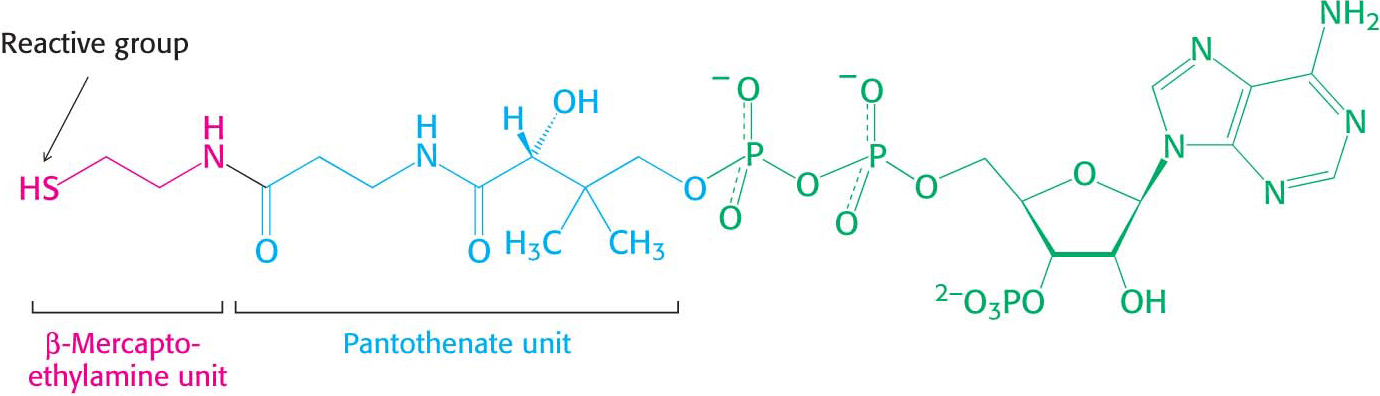
An Activated Carrier of Two-
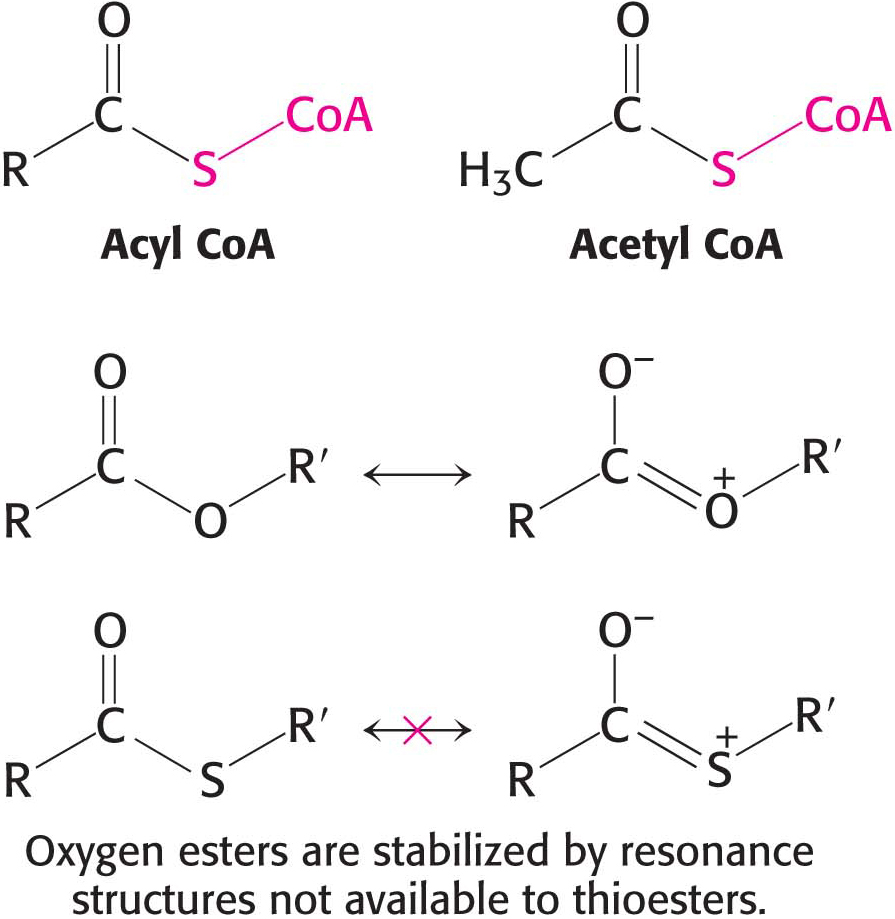
Carbon Fragments . Coenzyme A (also called CoA-SH), another central molecule in metabolism, is a carrier of acyl groups (Figure 15.17). A key constituent of coenzyme A is the vitamin pantothenate. Acyl groups are important constituents both in catabolism, as in the oxidation of fatty acids, and in anabolism, as in the synthesis of membrane lipids. The terminal sulfhydryl group in CoA is the reactive site. Acyl groups are linked to the sulfhydryl group of CoA by thioester bonds. The resulting derivative is called an acyl CoA. An acyl group often linked to CoA is the acetyl unit; this derivative is called acetyl CoA. The ΔG°′ for the hydrolysis of acetyl CoA has a large negative value: 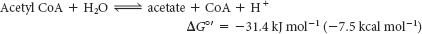
The hydrolysis of a thioester is thermodynamically more favorable than that of an oxygen ester, such as those in fatty acids, because the electrons of the C=O bond form less stable resonance structures with the C–
Additional features of activated carriers are responsible for two key aspects of metabolism. First, NADH, NADPH, and FADH2 react slowly with O2 in the absence of a catalyst. Likewise, ATP and acetyl CoA are hydrolyzed slowly (in times of many hours or even days) in the absence of a catalyst. These molecules are kinetically quite stable in the face of a large thermodynamic driving force for reaction with O2 (in regard to the electron carriers) and H2O (for ATP and acetyl CoA). The kinetic stability of these molecules in the absence of specific catalysts is essential for their biological function because it enables enzymes to control the flow of free energy and reducing power.
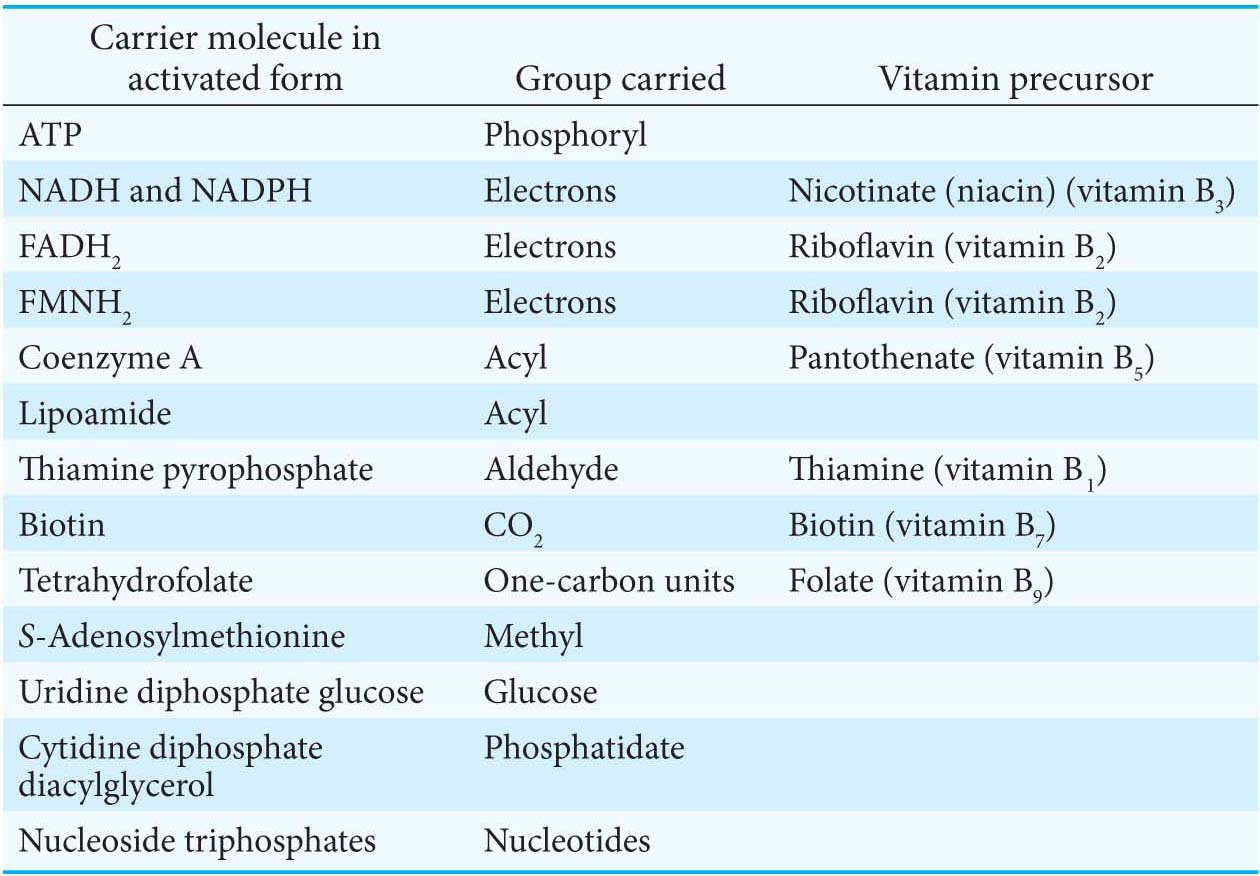
Second, most interchanges of activated groups in metabolism are accomplished by a rather small set of carriers (Table 15.2). The existence of a recurring set of activated carriers in all organisms is one of the unifying motifs of biochemistry.
NUTRITION FACTS: Pantothenate

 CLINICAL INSIGHT
CLINICAL INSIGHTLack of Activated Pantothenate Results in Neurological Problems
Pantothenate kinase associated degeneration, formerly called Hallervorden–
An important regulatory enzyme in the biosynthetic pathway for coenzyme A, pantothenate kinase activates the vitamin pantothenate by phosphorylating it at the expense of ATP.
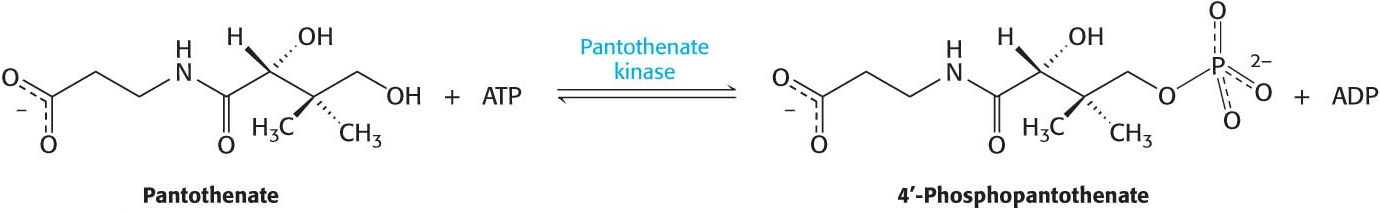
In subsequent steps, pantothenate phosphate is converted into coenzyme A. Presumably, the symptoms of pantothenate kinase associated degeneration are predominately neurological because the nervous system is absolutely dependent on aerobic metabolism, and coenzyme A is an essential player in the aerobic metabolism of all fuels (as we will see in Sections 8 and 9).
Many activated Carriers are Derived from Vitamins
Almost all the activated carriers that act as coenzymes are derived from vitamins—organic molecules needed in small amounts in the diets of many higher animals. Table 15.3 lists the vitamins that act as coenzymes. The series of vitamins known as the vitamin B group is shown in Figure 15.18. Note that, in all cases, the vitamin must be modified before it can serve its function (Appendix D). We have already touched on the roles of niacin, riboflavin, and pantothenate. These three and the other B vitamins will appear many times in our study of biochemistry.
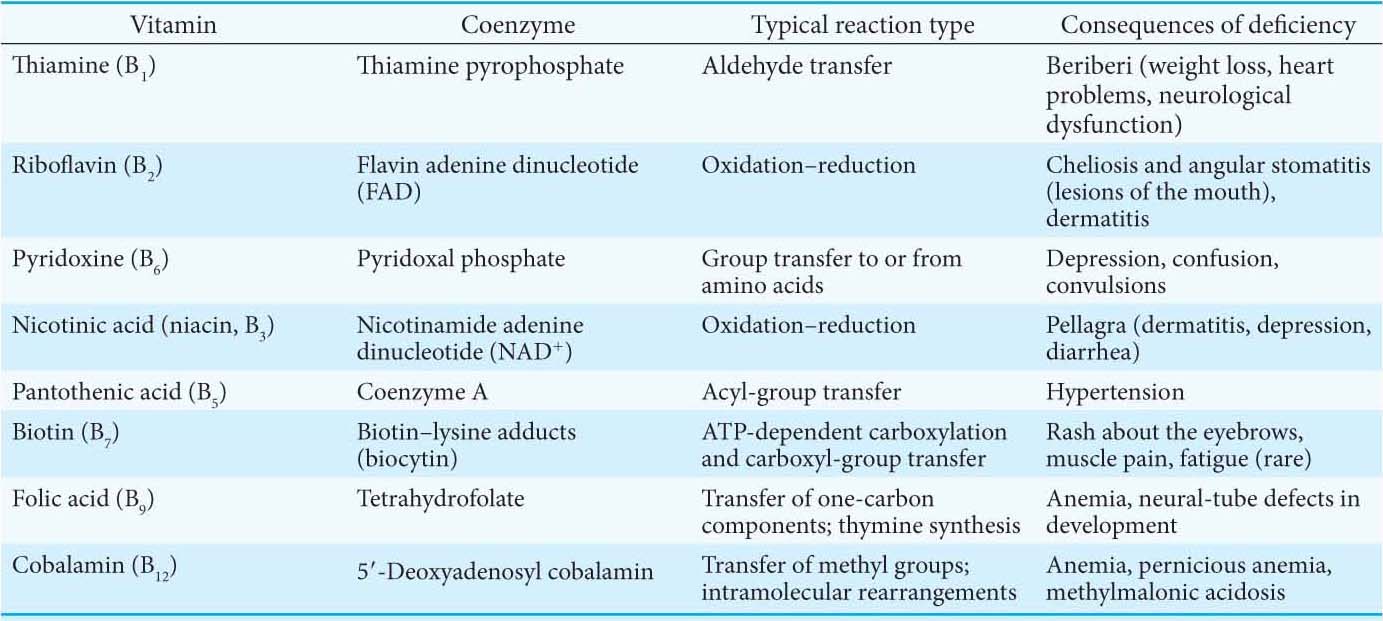
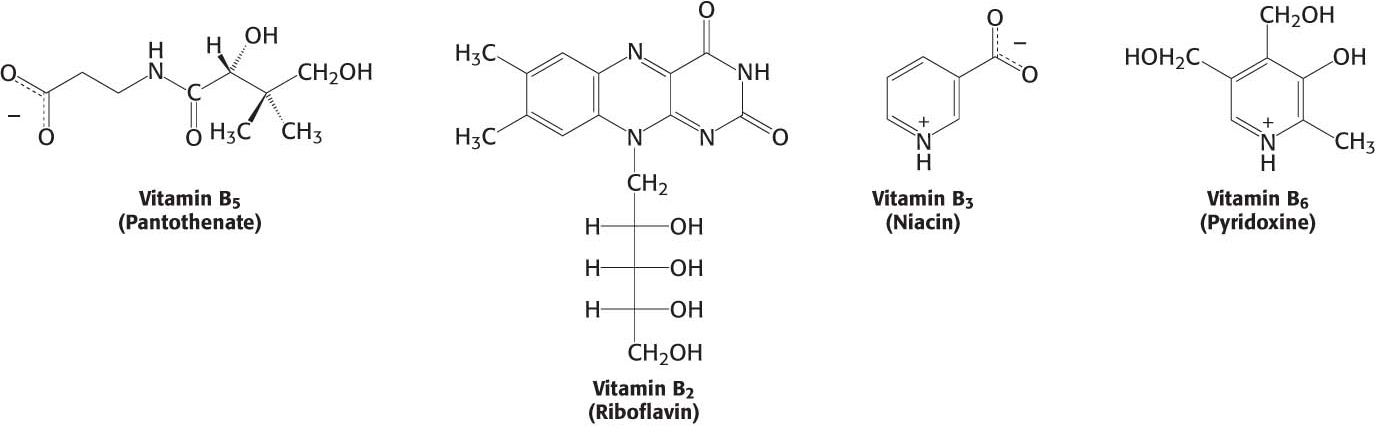
Vitamins serve the same important roles in nearly all forms of life, but higher animals lost the capacity to synthesize them in the course of evolution. For instance, whereas E. coli can thrive on glucose and organic salts, human beings require at least 12 vitamins in their diet. The biosynthetic pathways for vitamins can be complex; thus, it is biologically more efficient to ingest vitamins than to synthesize the enzymes required to construct them from simple molecules. This efficiency comes at the cost of dependence on other organisms for chemicals essential for life. Indeed, vitamin deficiency can generate diseases in all organisms requiring these molecules (Table 15.4; see also Table 15.3).
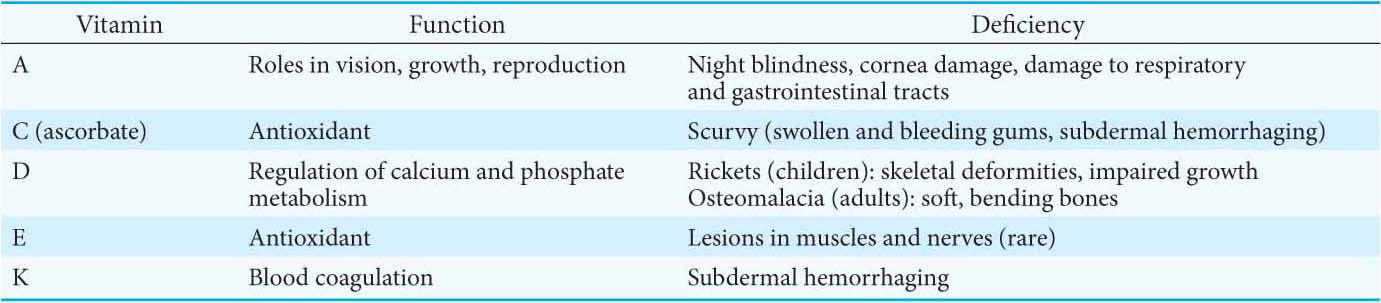
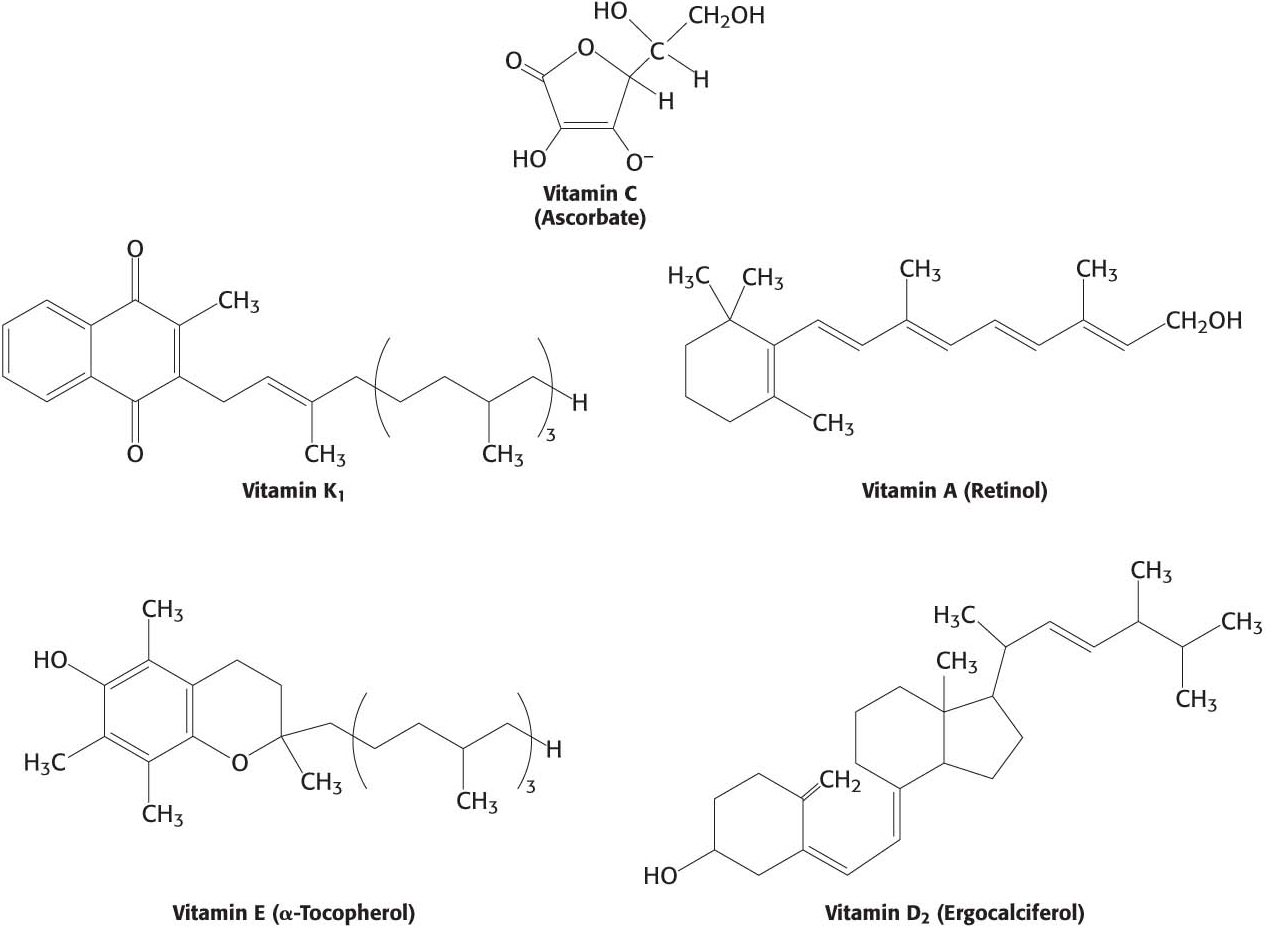
Not all vitamins function as coenzymes. Vitamins designated by the letters A, C, D, E, and K (Figure 15.19; Table 15.4) have a diverse array of functions. Vitamin A (retinol) is the precursor of retinal, the light-