
16.4 The Glycolytic Pathway Is Tightly Controlled
The glycolytic pathway has a dual role: it degrades glucose to generate ATP, and it provides building blocks for biosynthetic reactions, such as the formation of fatty acids and amino acids. The rate of conversion of glucose into pyruvate is regulated to meet these two major cellular needs. In metabolic pathways, enzymes catalyzing irreversible reactions are potential sites of control. In glycolysis, the reactions catalyzed by hexokinase, phosphofructokinase, and pyruvate kinase are irreversible, and each of them serves as a control site. These enzymes become more active or less so in response to the reversible binding of allosteric effectors or covalent modification. We will consider the control of glycolysis in two different tissues—
Glycolysis in Muscle Is Regulated by Feedback Inhibition to Meet the Need for ATP
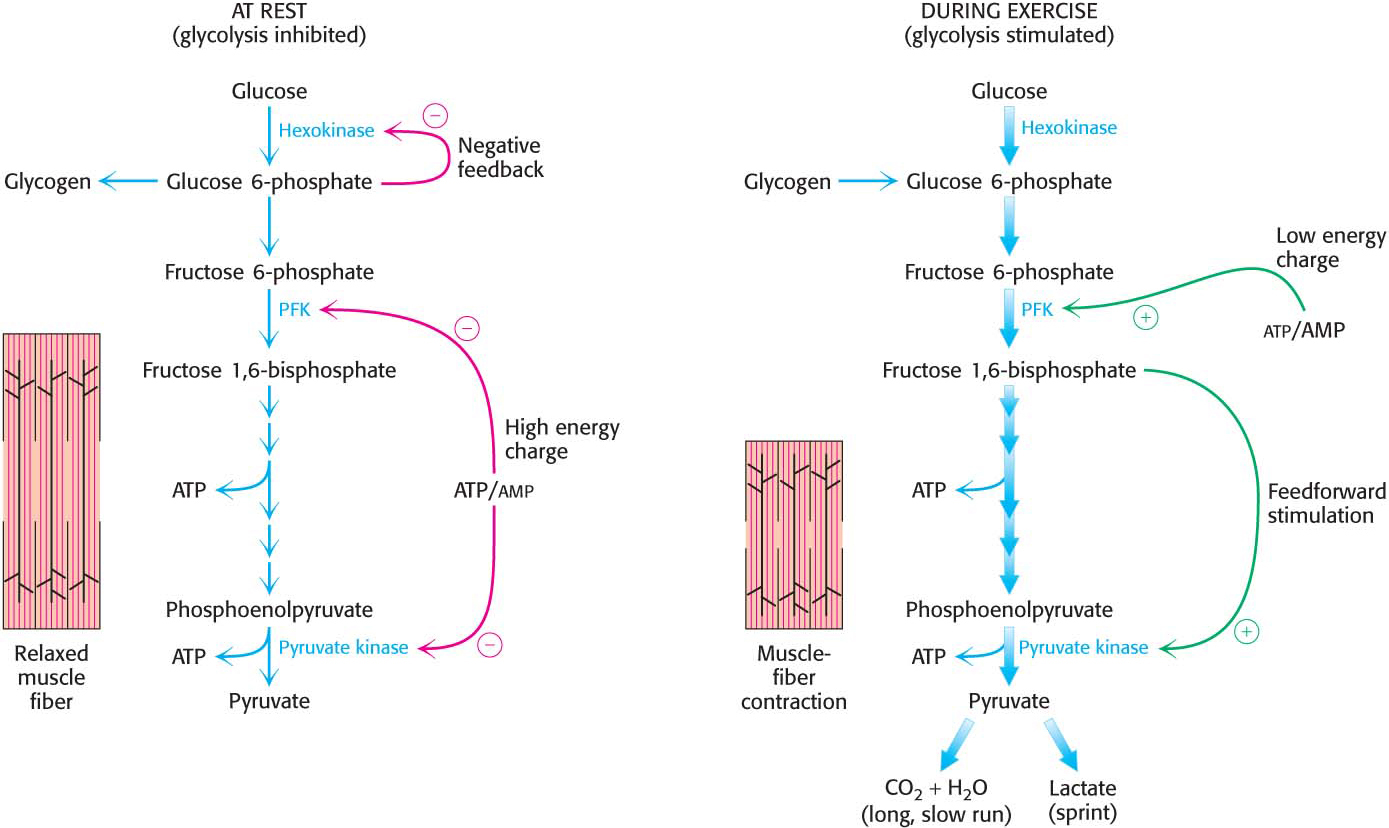
Glycolysis in skeletal muscle provides ATP primarily to power contraction. Consequently, the primary control of muscle glycolysis is the energy charge of the cell—
PhosphofructokinasePhosphofructokinase is the most important control site in the mammalian glycolytic pathway. High levels of ATP allosterically inhibit the enzyme (a 340-
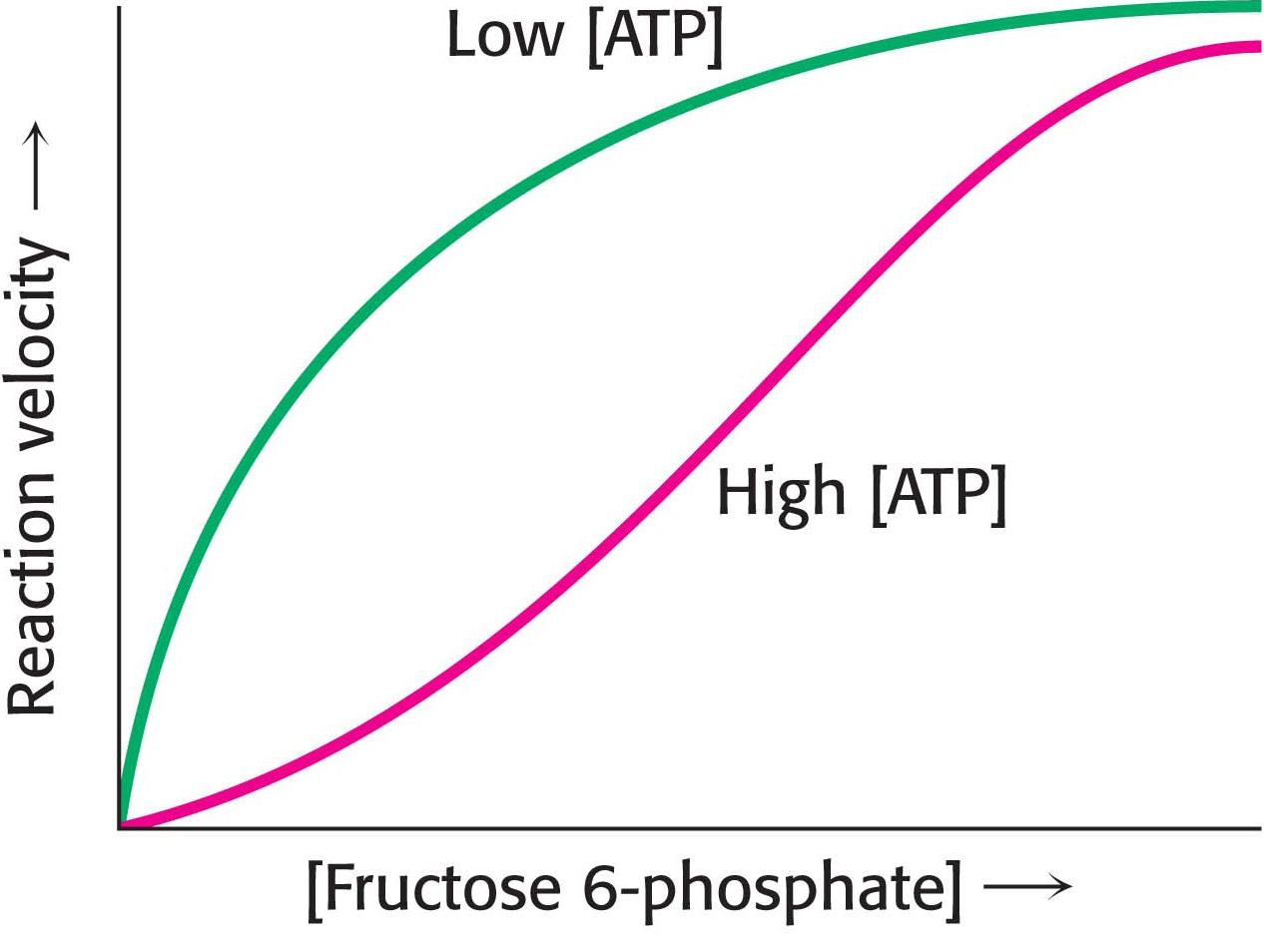
Why does AMP but not ADP stimulate the activity of phosphofructokinase? When ATP is being utilized rapidly, the enzyme adenylate kinase can form ATP from ADP by the following reaction:

Thus, some ATP is salvaged from ADP, and AMP becomes the signal for the low-
HexokinasePhosphofructokinase is the primary regulatory enzyme in glycolysis, but it is not the only one. Hexokinase, the enzyme catalyzing the first step of glycolysis, is inhibited by its product, glucose 6-
Why is phosphofructokinase rather than hexokinase the pacemaker of glycolysis? The reason becomes evident on noting that glucose 6-
Pyruvate kinasePyruvate kinase, the enzyme catalyzing the third irreversible step in glycolysis, controls the efflux from this pathway. This final step yields ATP and pyruvate, a central metabolic intermediate that can be oxidized further or used as a building block. ATP allosterically inhibits pyruvate kinase to decrease the rate of glycolysis when the energy charge of the cell is high. When the pace of glycolysis increases, fructose 1,6-
The Regulation of Glycolysis in the Liver Corresponds to the Biochemical Versatility of the Liver
The liver has a greater diversity of biochemical functions than muscle. Significantly, the liver maintains blood-
PhosphofructokinaseLiver phosphofructokinase can be regulated by ATP as in muscle, but such regulation is not as important since the liver does not experience the sudden ATP needs that a contracting muscle does. Likewise, low pH is not a metabolic signal for the liver enzyme, because lactate is not normally produced in the liver. Indeed, as we will see, lactate is converted into glucose in the liver.
Glycolysis in the liver furnishes carbon skeletons for biosyntheses, and so a signal indicating whether building blocks are abundant or scarce should also regulate phosphofructokinase. In the liver, phosphofructokinase is inhibited by citrate, an early intermediate in the citric acid cycle (Chapter 19). A high level of citrate in the cytoplasm means that biosynthetic precursors are abundant, and so there is no need to degrade additional glucose for this purpose. In this way, citrate enhances the inhibitory effect of ATP on phosphofructokinase.
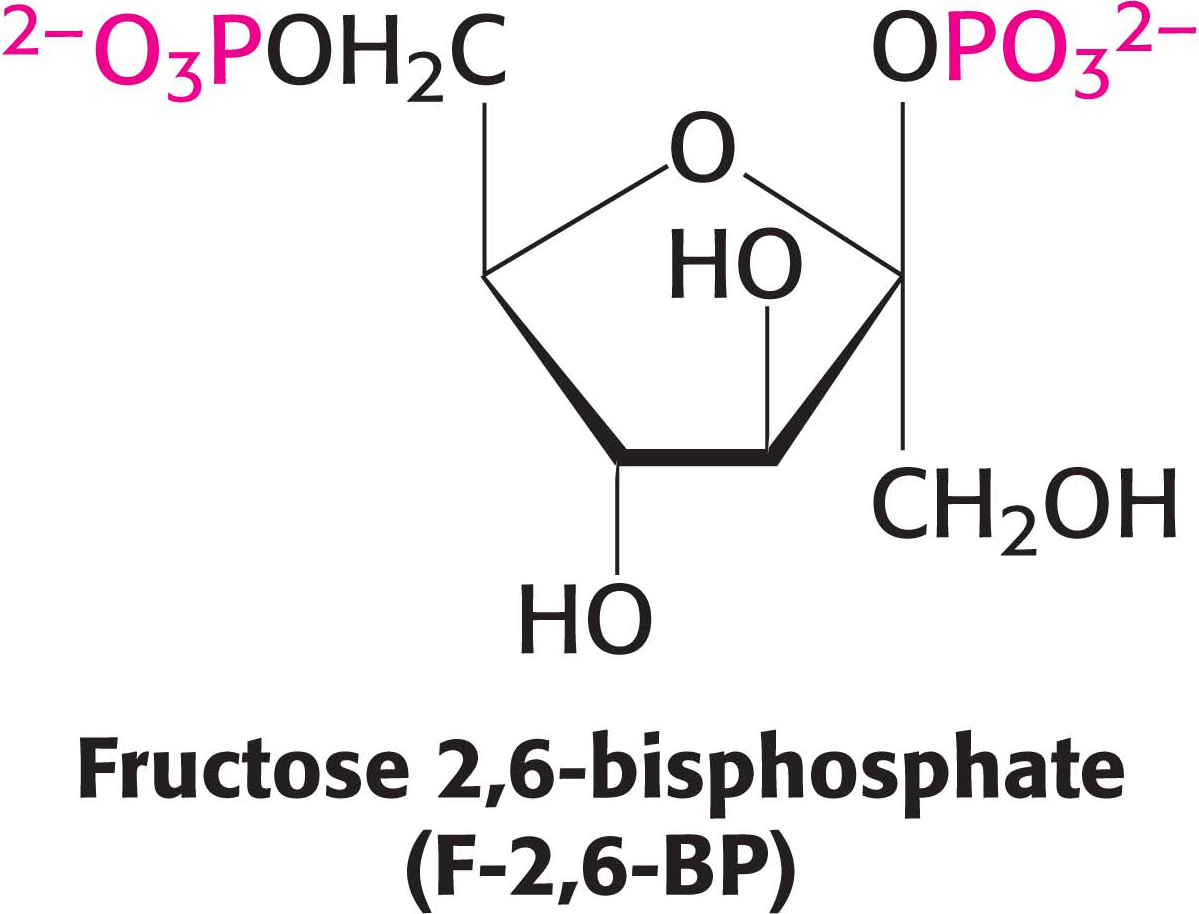
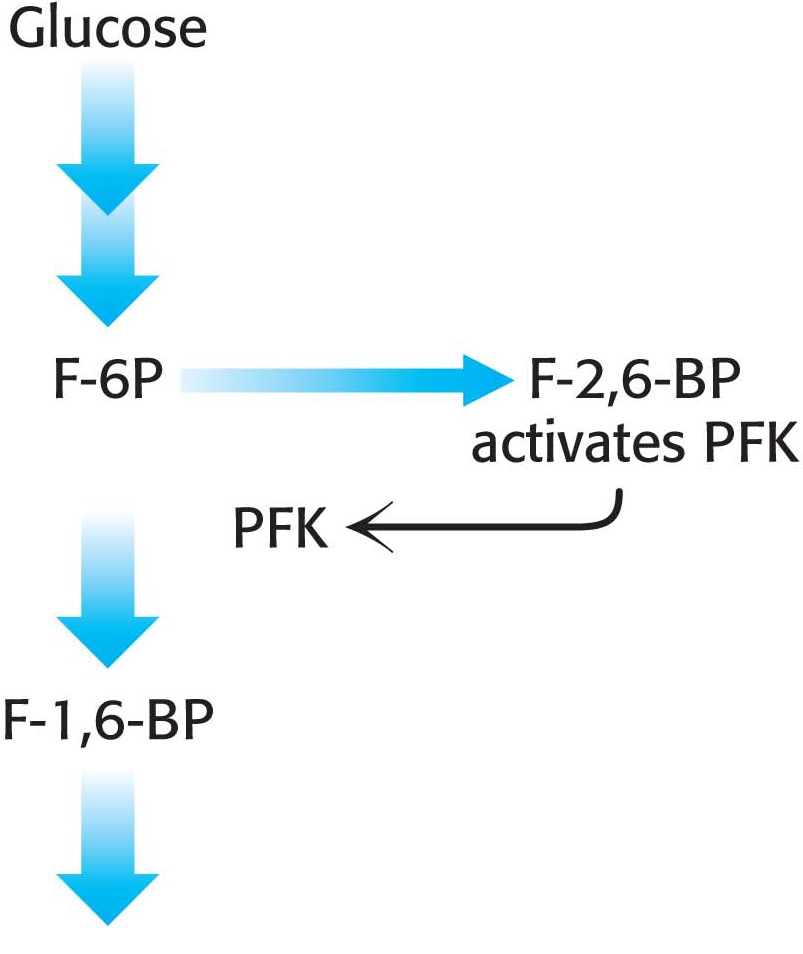
The key means by which glycolysis in the liver responds to changes in blood glucose is through the signal molecule fructose 2,6-
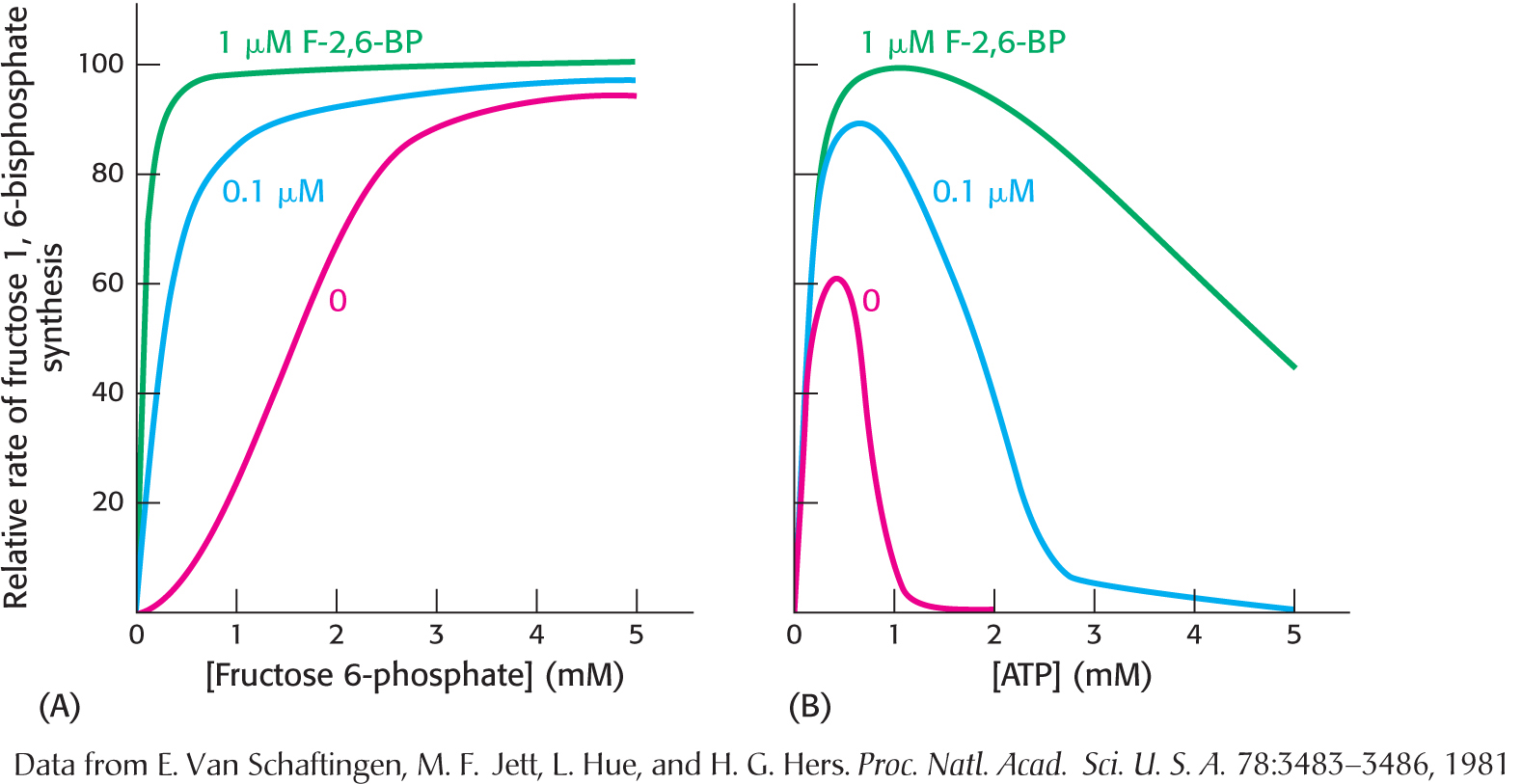
Hexokinase and glucokinaseIn the liver as well as in muscle, hexokinase is a regulatory enzyme. The hexokinase reaction is controlled in the liver as in muscle. However, the enzyme primarily responsible for phosphorylating glucose in the liver is not hexokinase, but glucokinase (hexokinase IV), an isozyme of hexokinase. Isozymes, or isoenzymes, are enzymes encoded by different genes with different amino acid sequences, yet they catalyze the same reaction. Isozymes usually differ in kinetic or regulatory properties. Glucokinase phosphorylates glucose only when glucose is abundant, as would be the case after a meal. The reason is that glucokinase’s KM for glucose is about 50-
Glucokinase is also present in the ß cells of the pancreas, where the increased formation of glucose 6-
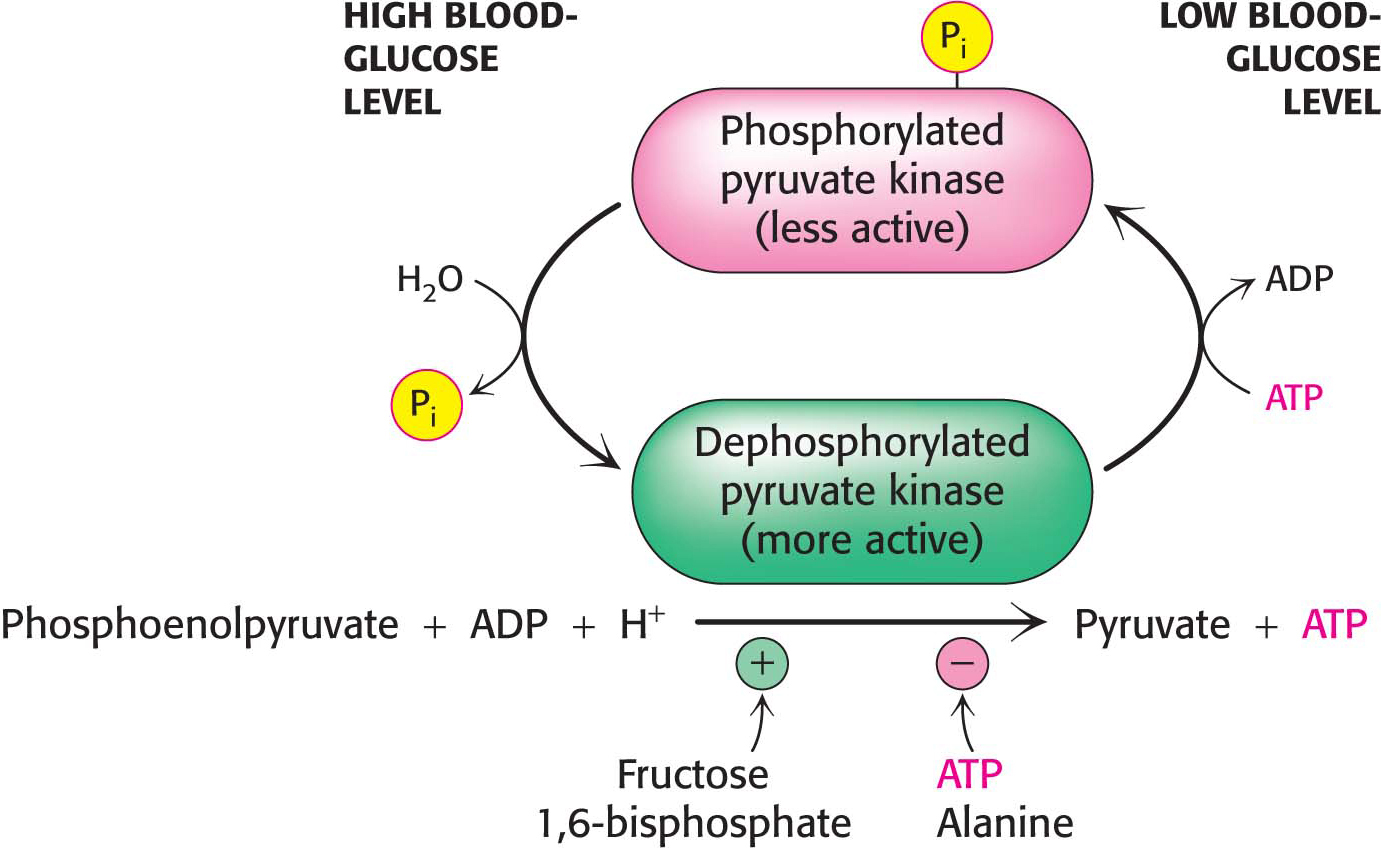
Pyruvate kinaseSeveral isozymic forms of pyruvate kinase (a tetramer of 57-
A Family of Transporters Enables Glucose to Enter and Leave Animal Cells
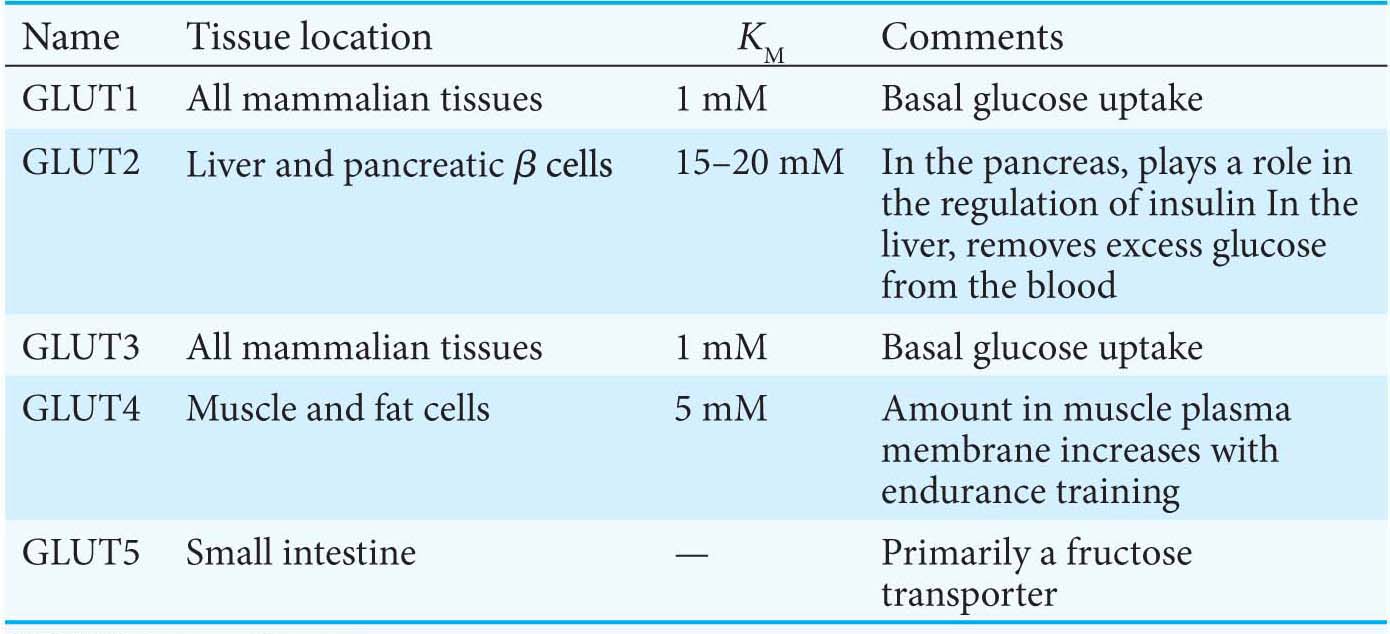
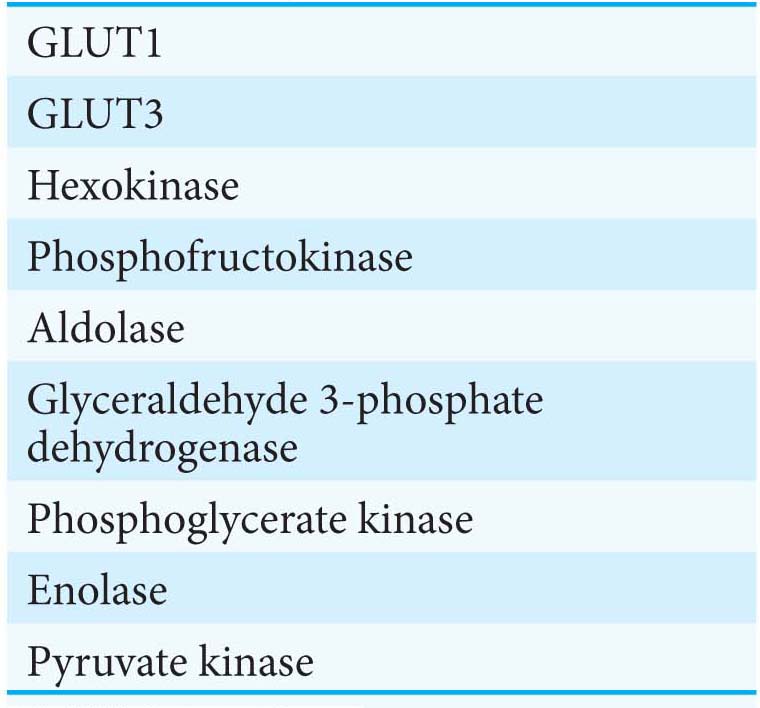
Several glucose transporters mediate the thermodynamically downhill movement of glucose across the plasma membranes of animal cells. Each member of this protein family, named GLUT1 to GLUT5, consists of a single polypeptide chain of about 500 amino acids (Table 16.3).
The members of this family have distinctive roles:
GLUT1 and GLUT3, present in nearly all mammalian cells, are responsible for transporting glucose into the cell under normal conditions. Like enzymes, transporters have KM values, except that, for transporters, KM is the concentration of the chemical transported that yields one-
half maximal transport velocity. The KM value for glucose for GLUT1 and GLUT3 is about 1 mM, significantly less than the normal serum- glucose concentration, which typically ranges from 4 mM to 8 mM. Hence, GLUT1 and GLUT3 continuously transport glucose into cells at an essentially constant rate. GLUT2, present in liver and pancreatic ß cells, is distinctive in having a very high KM value for glucose (15–
20 mM). Hence, glucose enters these tissues at a biologically significant rate only when there is much glucose in the blood. The pancreas can thereby sense the glucose level and adjust the rate of insulin secretion accordingly. Insulin signals the need to remove glucose from the blood for storage as glycogen or conversion into fat (Chapters 25 and 28). The high KM value of GLUT2 also ensures that glucose rapidly enters liver cells only in times of plenty. GLUT4, which has a KM value of 5 mM, transports glucose into muscle and fat cells. The number of GLUT4 transporters in the plasma membrane increases rapidly in the presence of insulin, which signals the presence of glucose in the blood. Hence, insulin promotes the uptake of glucose by muscle and fat. Endurance exercise training also increases the amount of GLUT4 present in muscle membranes by a means independent of insulin.
GLUT5, present in the small intestine, functions primarily as a fructose transporter.
 CLINICAL INSIGHT
CLINICAL INSIGHTAerobic Glycolysis Is a Property of Rapidly Growing Cells
Tumors have been known for decades to display enhanced rates of glucose uptake and glycolysis. Indeed, rapidly growing tumor cells will metabolize glucose to lactate even in the presence of oxygen, a process called aerobic glycolysis or the “Warburg effect,” after Otto Warburg, the biochemist who first noted this characteristic of cancer cells in the 1920s. In fact, tumors with a high glucose uptake are particularly aggressive, and the cancer is likely to have a poor prognosis. A nonmetabolizable glucose analog, 2-
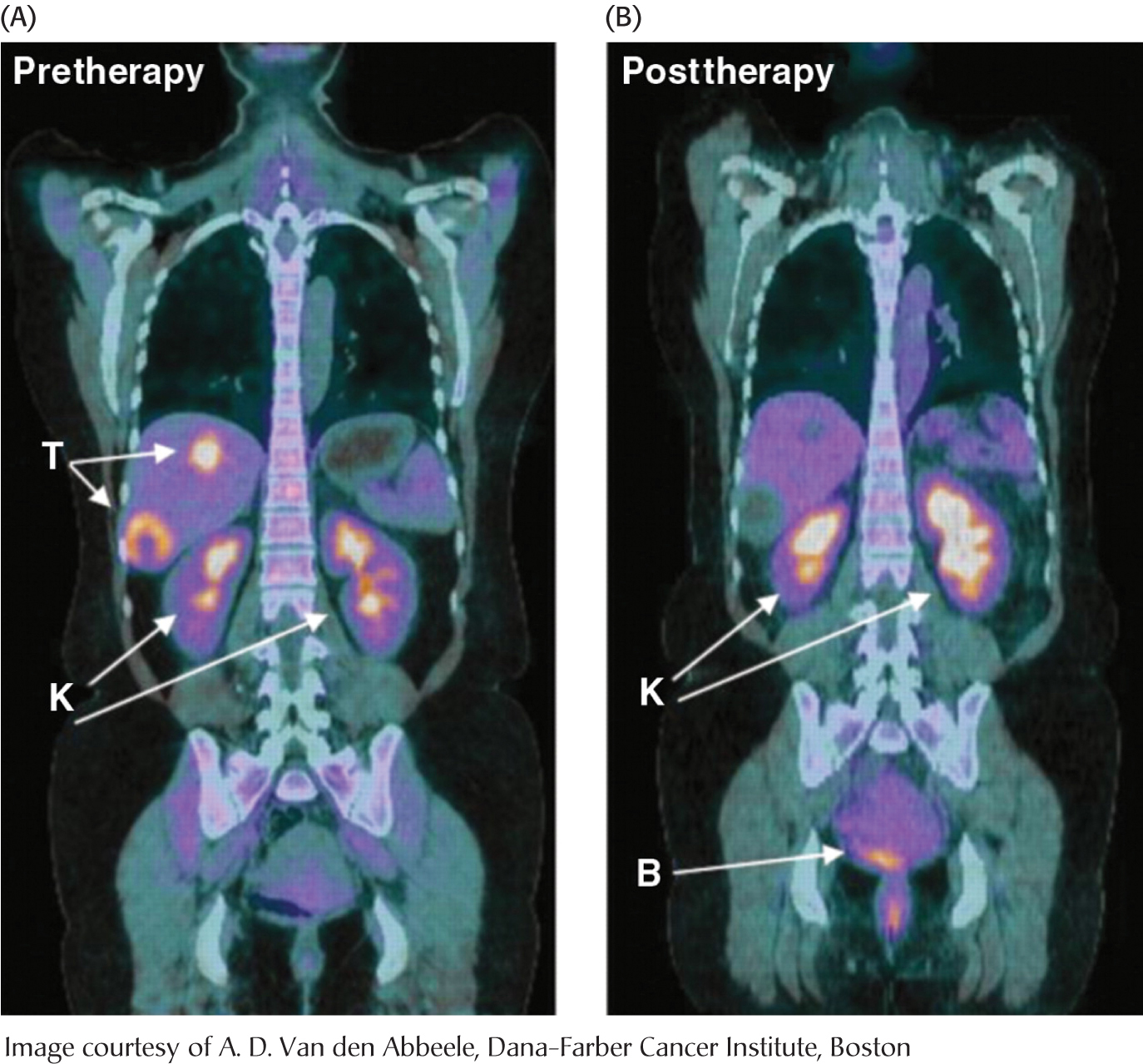
What selective advantage does aerobic glycolysis offer the tumor over the energetically more efficient oxidative phosphorylation? Research is being actively pursued to answer the question, but we can speculate on the benefits. First, aerobic glycolysis generates lactic acid that is then secreted. Acidification of the tumor environment has been shown to facilitate tumor invasion and inhibit the immune system from attacking the tumor. However, even leukemia cells perform aerobic glycolysis, and leukemia is not an invasive cancer. Second, and perhaps more importantly, the increased uptake of glucose and formation of glucose 6-
What biochemical alterations facilitate the switch to aerobic glycolysis? Again, the answers are not complete, but changes in gene expression of isozymic forms of two glycolytic enzymes may be crucial. Tumor cells express an isozyme of hexokinase that binds to mitochondria. There, the enzyme has ready access to any ATP generated by oxidative phosphorylation and is not susceptible to feedback inhibition by its product, glucose 6-
 CLINICAL INSIGHT
CLINICAL INSIGHTCancer and Exercise Training Affect Glycolysis in a Similar Fashion
The hypoxia that some tumors experience with rapid growth activates a transcription factor, hypoxia-
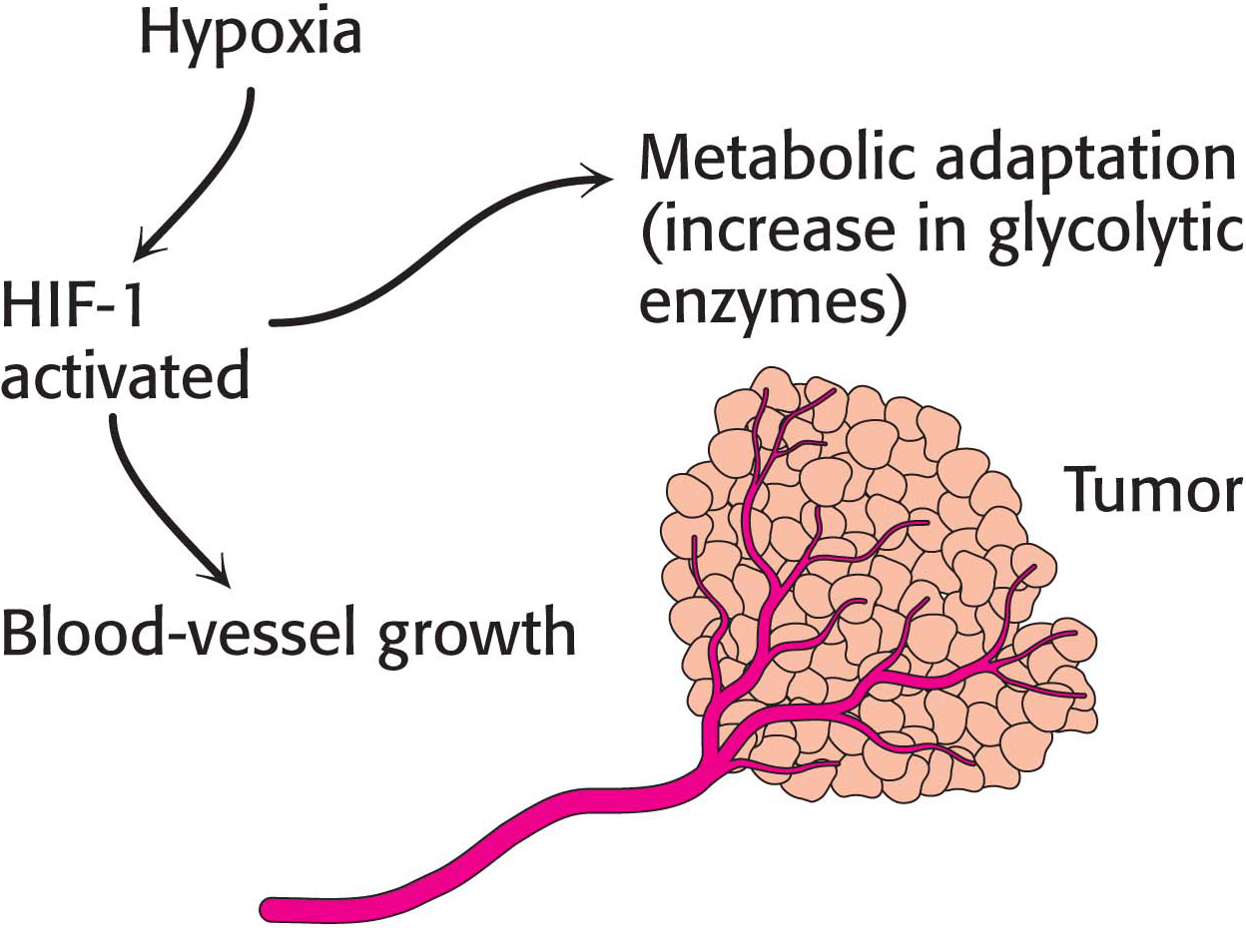
Interestingly, anaerobic exercise training activates HIF-