
19.3 Stage Two Regenerates Oxaloacetate and Harvests Energy-Rich Electrons
The second part of the citric acid cycle consists of the regeneration of the starting material, oxaloacetate. This regeneration is accomplished by a series of reactions that begins with a four-
A Compound with High Phosphoryl-Transfer Potential Is Generated from Succinyl Coenzyme A
The succinyl CoA produced by α-ketoglutarate dehydrogenase in the preceding step is an energy-
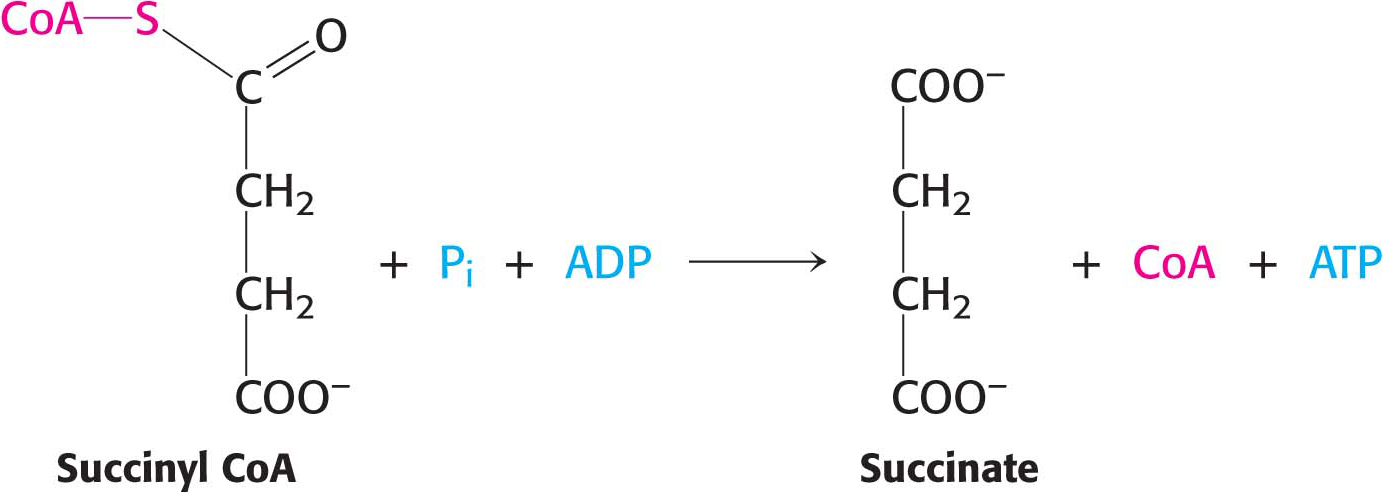
This reaction is the only step in the citric acid cycle that directly yields a compound with high phosphoryl-
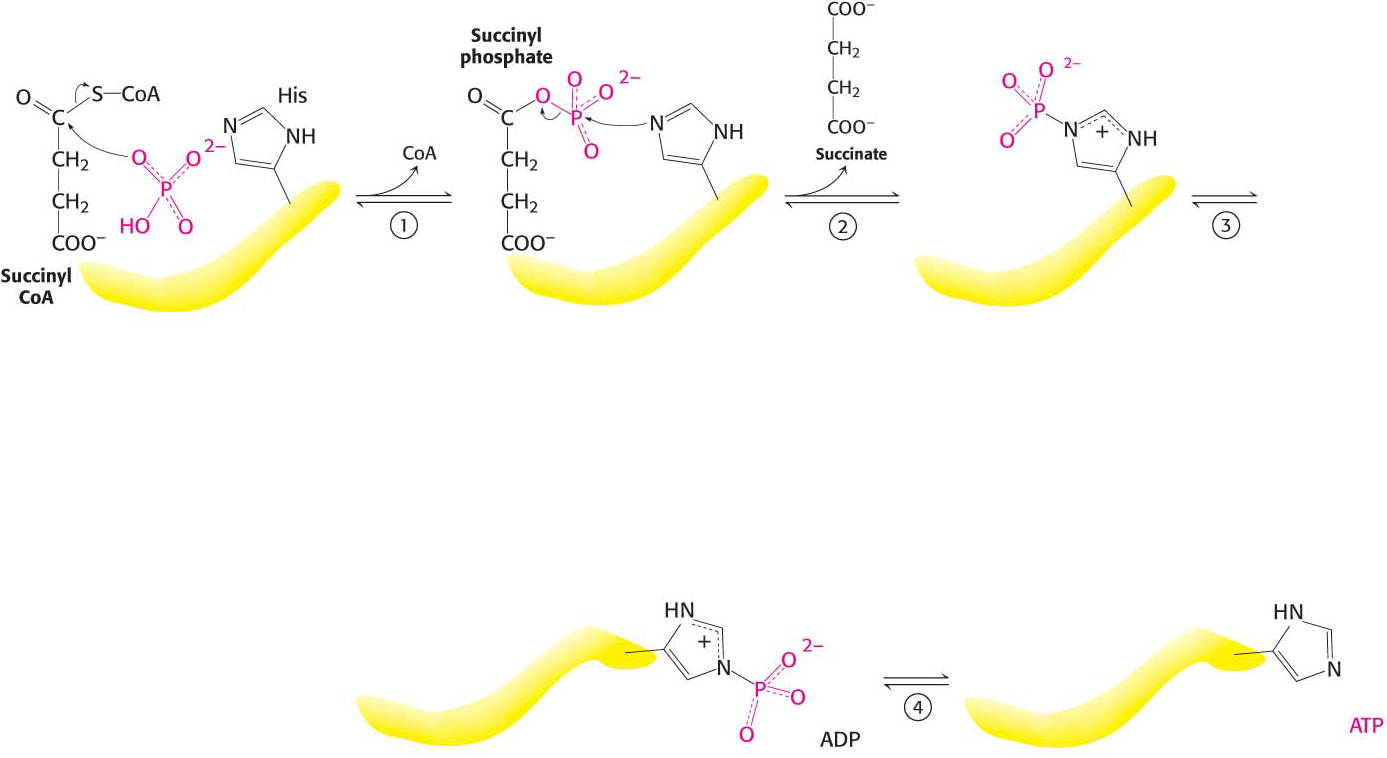
Succinyl Coenzyme A Synthetase Transforms Types of Biochemical Energy
The mechanism of this reaction is a clear example of an energy transformation: energy inherent in the thioester is transformed into phosphoryl-
Oxaloacetate Is Regenerated by the Oxidation of Succinate
Succinate is subsequently oxidized to regenerate oxaloacetate.
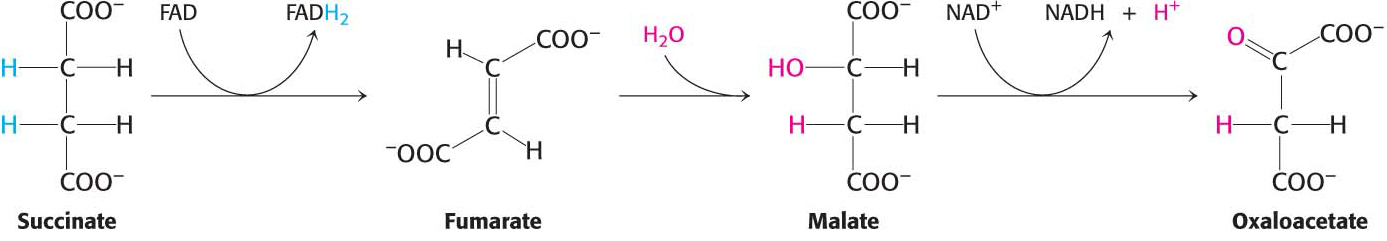
The reactions constitute a metabolic motif that we will see again in fatty acid degradation (Chapter 27) and synthesis (Chapter 28). A methylene group (CH2) is converted into a carbonyl group (C=O) in three steps: an oxidation, a hydration, and a second oxidation reaction. Oxaloacetate is thereby regenerated for another round of the cycle, and more energy is extracted in the form of FADH2 and NADH.
The first step in this set of reactions, the oxidation of succinate to fumarate, is catalyzed by succinate dehydrogenase. The hydrogen acceptor is FAD rather than NAD+, which is used in the other three oxidation reactions in the cycle. FAD is the hydrogen acceptor in this reaction because the free-
Succinate dehydrogenase differs from other enzymes in the citric acid cycle because it is embedded in the inner mitochondrial membrane in association with the electron-
After succinate has been oxidized to fumarate, the next step is the hydration of fumarate to form l-
DID YOU KNOW?
Apples are a rich source of malic acid, which used to be called “acid of apples.” In fact, the word malic is derived from the Latin malum, meaning “apple.”
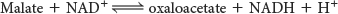
The standard free energy for this reaction, unlike that for the other steps in the citric acid cycle, is significantly positive ΔG°′ = +29.7 kJ mol−1, or +7.1 kcal mol−1). The oxidation of malate is driven by the use of the products—
The Citric Acid Cycle Produces High-Transfer-Potential Electrons, an ATP, and Carbon Dioxide
The net reaction of the citric acid cycle is
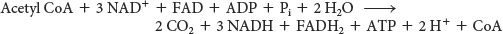
Let us review the reactions that give this stoichiometry (Figure 19.6 and Table 19.1):
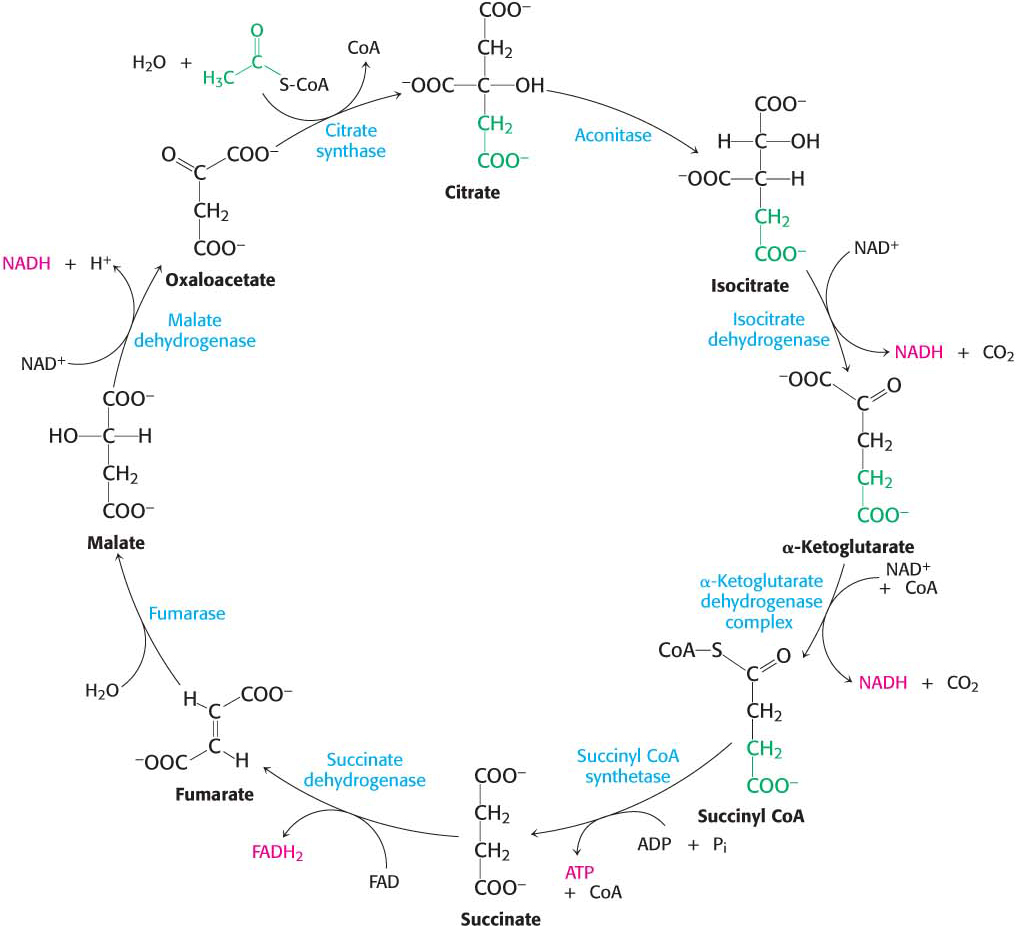
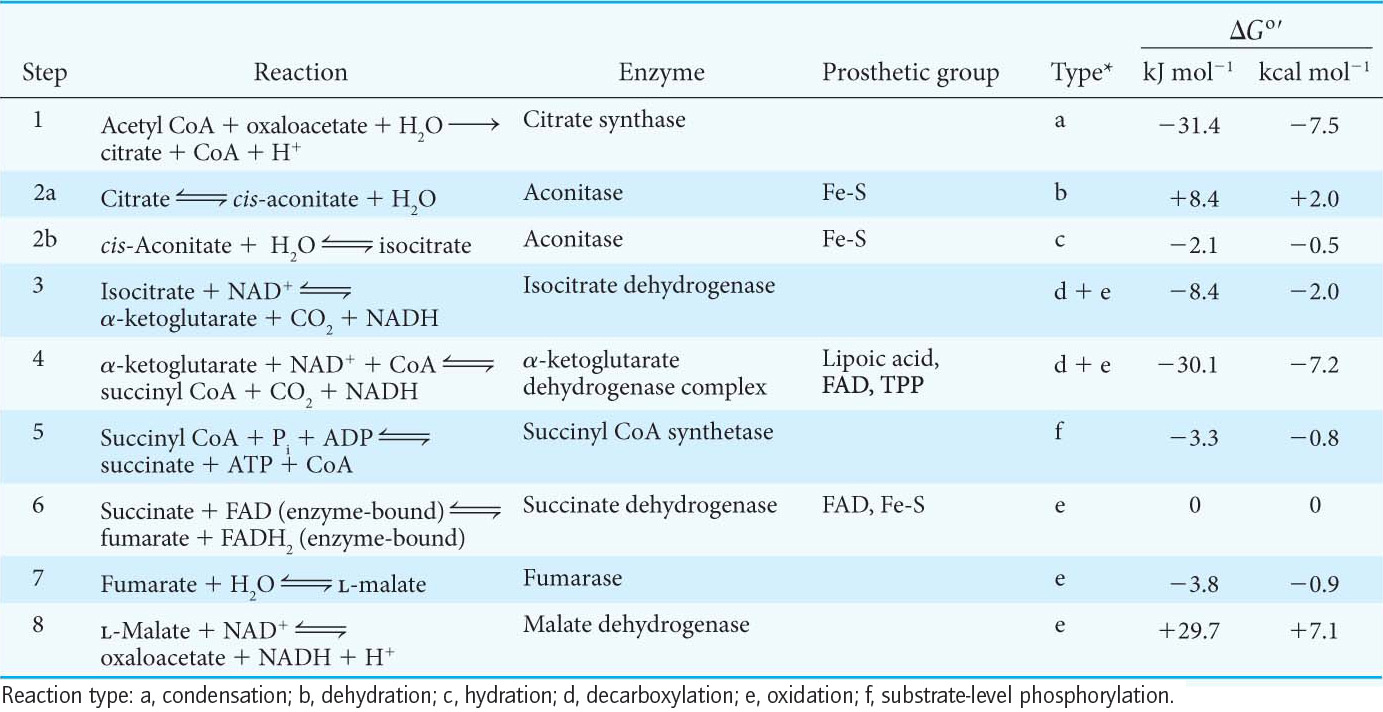
Two carbon atoms enter the cycle in the condensation of an acetyl unit (from acetyl CoA) with oxaloacetate. Two carbon atoms leave the cycle in the form of CO2 in the successive decarboxylations catalyzed by isocitrate dehydrogenase and α-ketoglutarate dehydrogenase.
Four pairs of hydrogen atoms leave the cycle in four oxidation reactions. Two NAD+ molecules are reduced in the oxidative decarboxylations of isocitrate and α-ketoglutarate, one FAD molecule is reduced in the oxidation of succinate, and one NAD+ molecule is reduced in the oxidation of malate. Recall also that one NAD+ molecule is reduced in the oxidative decarboxylation of pyruvate to form acetyl CoA.
One ATP is generated from the cleavage of the thioester linkage in succinyl CoA.
Two water molecules are consumed: one in the synthesis of citrate by the hydrolysis of citryl CoA and the other in the hydration of fumarate.
Isotope-
DID YOU KNOW?
The manuscript proposing the citric acid cycle was submitted for publication to Nature but was rejected. Dr. Hans Krebs proudly displayed the rejection letter of June 1937 throughout his career as encouragement for young scientists. His work was subsequently published in Enzymologia.
QUICK QUIZ 1
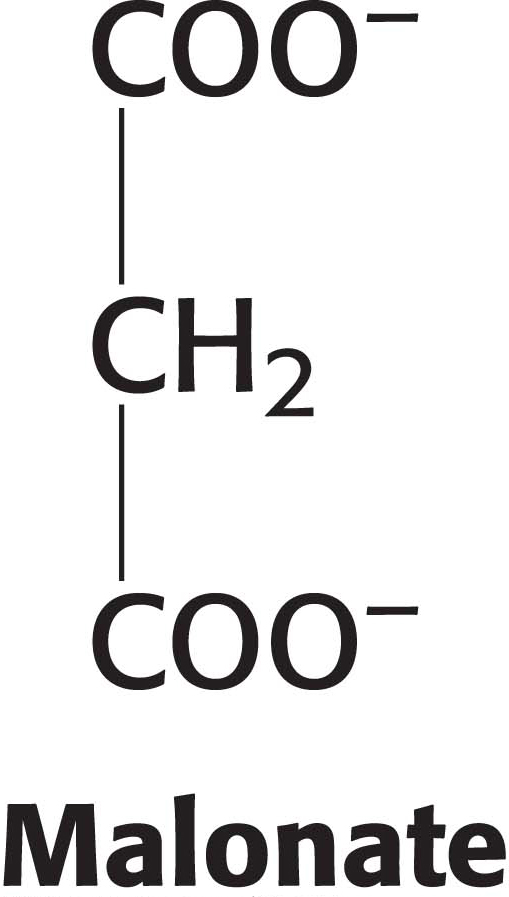
Malonate is a competitive inhibitor of succinate dehydrogenase. How will the concentrations of citric acid cycle intermediates change immediately after the addition of malonate? Why is malonate not a substrate for succinate dehydrogenase?
Succinate will increase in concentration, followed by α-ketoglutarate and the other intermediates “upstream” of the site of inhibition. Succinate has two methylene groups that are required for the dehydrogenation, whereas malonate has but one.
Evidence is accumulating that the enzymes of the citric acid cycle are physically associated with one another. The close arrangement of enzymes enhances the efficiency of the citric acid cycle because a reaction product can pass directly from one active site to the next through connecting channels, a process called substrate channeling.
The key catabolic function of the citric acid cycle is the production of high-
Recall that molecular oxygen does not participate directly in the citric acid cycle. However, the cycle operates only under aerobic conditions because NAD+ and FAD can be regenerated in mitochondria only by the transfer of electrons to molecular oxygen. Glycolysis has both an aerobic and an anaerobic mode, whereas the citric acid cycle is strictly aerobic. As discussed in Chapter 16, glycolysis can proceed under anaerobic conditions because NAD+ is regenerated in the conversion of pyruvate into lactate or ethanol.