
19.4 The Citric Acid Cycle Is Regulated
✓ 5 Describe how the citric acid cycle is regulated.
✓ 6 Describe the role of the citric acid cycle in anabolism.
The citric acid cycle is the final common pathway for the aerobic oxidation of fuel molecules. Moreover, as we will see shortly and repeatedly elsewhere in our study of biochemistry, the cycle is an important source of building blocks for a host of biomolecules. As befits its role as the metabolic hub of the cell, entry into the cycle and the rate of the cycle itself are controlled at several stages. We have seen that the pyruvate dehydrogenase complex, the link between glycolysis and the citric acid cycle, is a regulatory site controlling the metabolism of pyruvate by the citric acid cycle.
The Citric Acid Cycle Is Controlled at Several Points
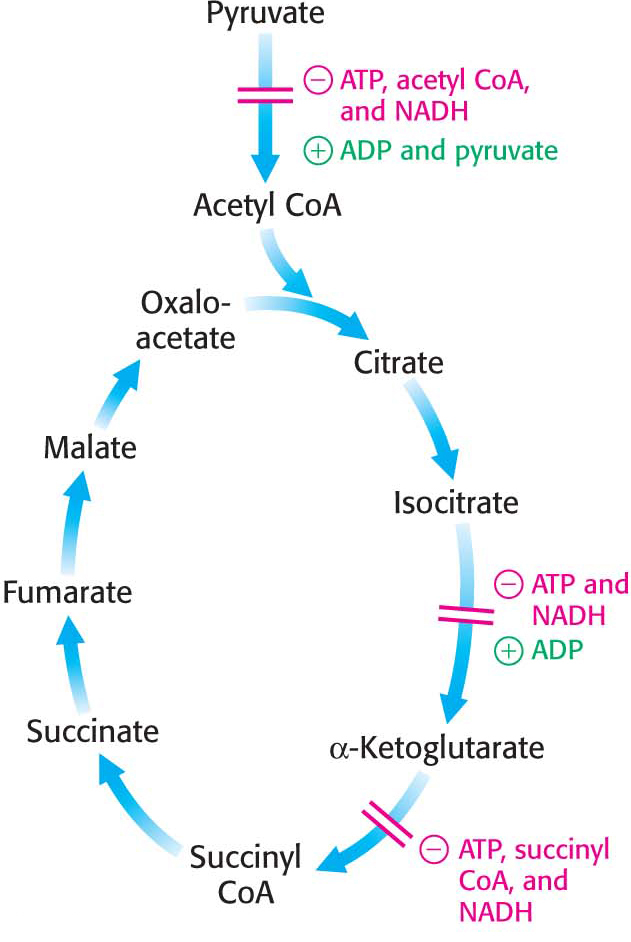
The regulation of pyruvate dehydrogenase is a crucial site of control, modulating the conversion of glucose-
For many tissues, such as the liver, the ATP needs are approximately constant on the time scale of minutes to hours. Thus, for these tissues, the citric acid cycle is operating at a constant rate. But, just as driving at a constant speed usually requires touching the gas pedal and the break occasionally, the rate of the citric acid cycle must be “tuned” with increases and decreases. What signals regulate the rate of the cycle? Isocitrate dehydrogenase is allosterically stimulated by ADP, which signifies the need for more energy. In contrast, NADH, which signals the presence of high-
A second control site in the citric acid cycle is α-ketoglutarate dehydrogenase, which catalyzes the rate-
Although different regulatory mechanisms might be expected in skeletal muscle, where the energy needs can vary immensely and rapidly, this does not appear to be the case. While the rate of the citric acid cycle will increase 100-
The use of isocitrate dehydrogenase and α-ketoglutarate dehydrogenase as control points integrates the citric acid cycle with other pathways and highlights the central role of the citric acid cycle in metabolism. For instance, the inhibition of isocitrate dehydrogenase leads to a buildup of citrate, because the interconversion of isocitrate and citrate is readily reversible under intracellular conditions. Citrate can be transported to the cytoplasm where it signals phosphofructokinase to halt glycolysis and where it can serve as a source of acetyl CoA for fatty acid synthesis (Chapter 28). The α-ketoglutarate that accumulates when α-ketoglutarate dehydrogenase is inhibited can be used as a precursor for several amino acids and the purine bases (Chapter 30).
In many bacteria, the funneling of two-
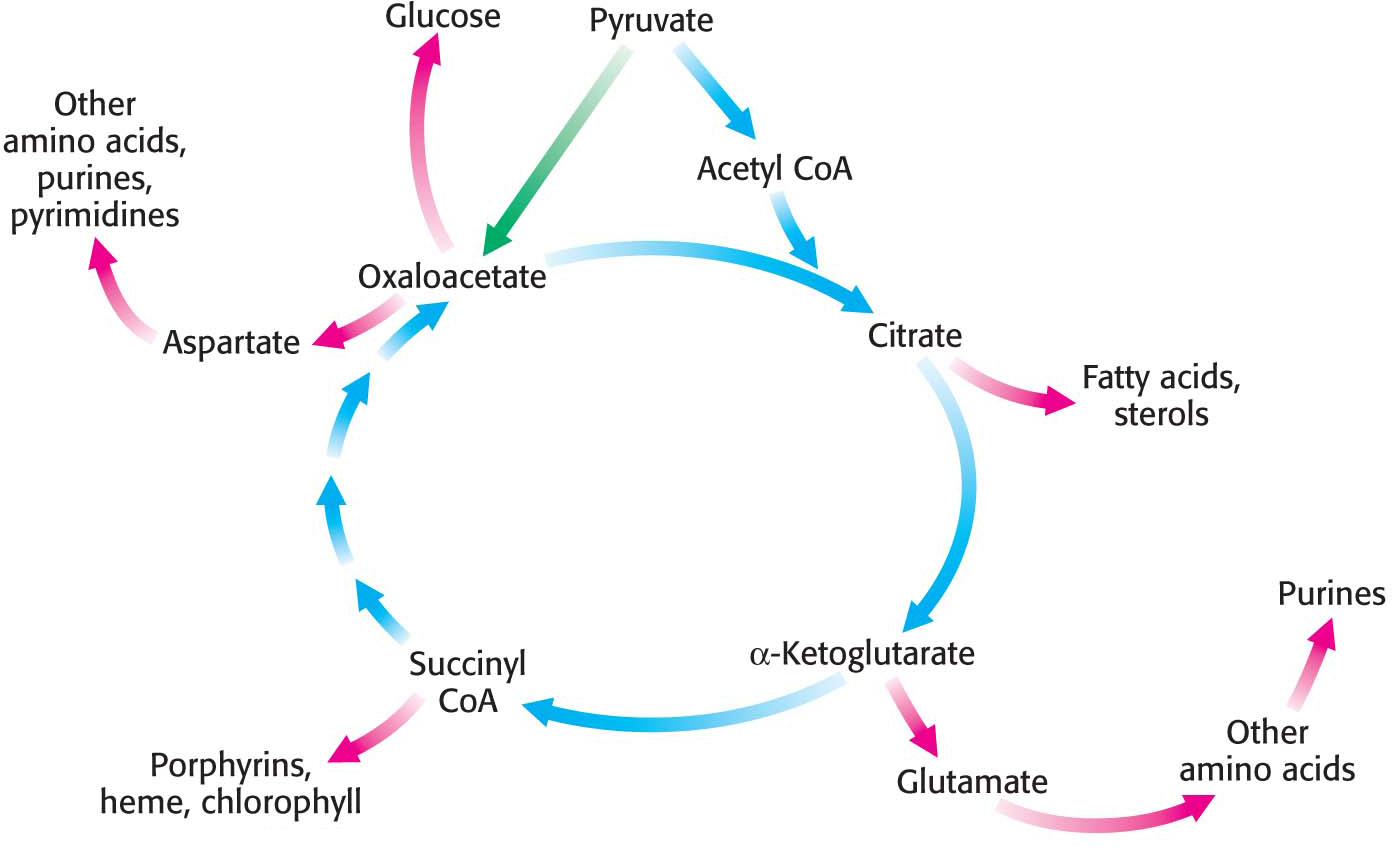
The Citric Acid Cycle Is a Source of Biosynthetic Precursors
Thus far, we have focused on the citric acid cycle as the major degradative pathway for the generation of ATP. As a major metabolic hub of the cell, the citric acid cycle also provides intermediates for biosyntheses (Figure 19.8). For example, most of the carbon atoms in porphyrins, the precursors to the heme groups in hemoglobin and myoglobin, come from succinyl CoA. Many of the amino acids are derived from α-ketoglutarate and oxaloacetate. These biosynthetic processes will be considered in subsequent chapters.
The Citric Acid Cycle Must Be Capable of Being Rapidly Replenished
The citric acid cycle is crucial for generating biological energy and is a source of building blocks for biosynthetic reactions. This dual use presents a problem. Suppose that much oxaloacetate is converted into glucose and, subsequently, the energy needs of the cell rise. The citric acid cycle will operate at a reduced capacity unless new oxaloacetate is formed, because acetyl CoA cannot enter the cycle unless it condenses with oxaloacetate. Even though oxaloacetate is recycled, a minimal level must be maintained to allow the cycle to function. Citric acid cycle intermediates must be replenished if any are drawn off for biosyntheses.
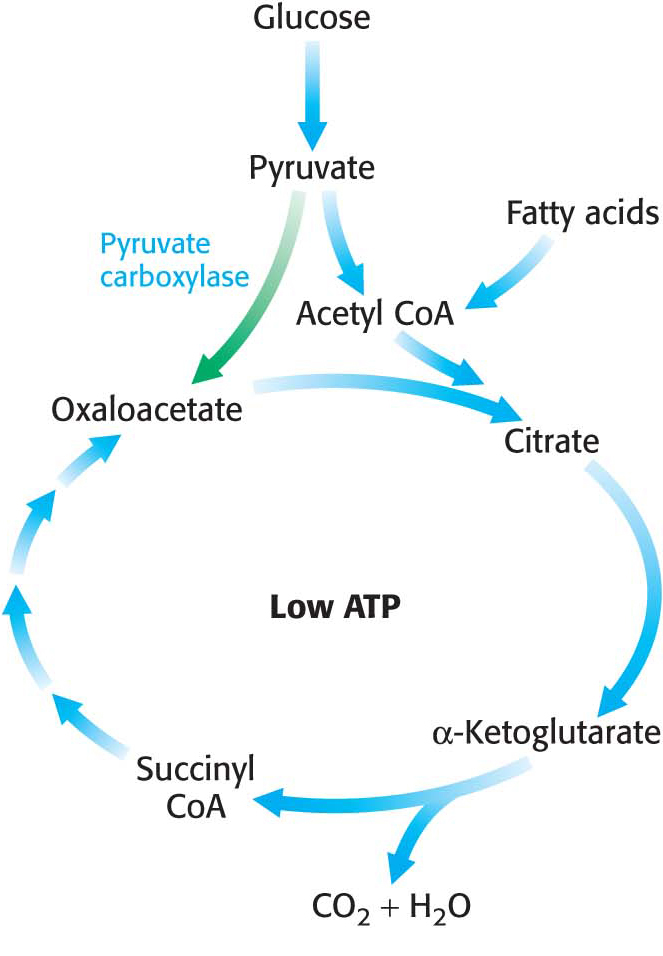
How is oxaloacetate replenished? Mammals lack the enzymes for the net conversion of acetyl CoA into oxaloacetate or any other citric acid cycle intermediate. Rather, oxaloacetate is formed by the carboxylation of pyruvate, in a reaction catalyzed by the biotin-

Recall that this enzyme plays a crucial role in gluconeogenesis. It is active only in the presence of acetyl CoA, which signifies the need for more oxaloacetate. If the energy charge is high, oxaloacetate is converted into glucose. If the energy charge is low, oxaloacetate replenishes the citric acid cycle. The synthesis of oxaloacetate by the carboxylation of pyruvate is an example of an anaplerotic reaction (of Greek origin, meaning to “fill up”), a reaction that leads to the net synthesis, or replenishment, of pathway components. Note that, because the citric acid cycle is a cycle, it can be replenished by the generation of any of the intermediates. For instance, the removal of the nitrogen groups from glutamate and aspartate yield α-ketoglutarate and oxaloacetate, respectively. Glutamine is an especially important source of citric acid cycle intermediates in rapidly growing cells, including cancer cells. Glutamine is converted into glutamate and then into α-ketoglutarate.
QUICK QUIZ 2
Why is acetyl CoA an especially appropriate activator for pyruvate carboxylase?
Pyruvate carboxylase should be active only when the acetyl CoA concentration is high. Acetyl CoA might accumulate if the energy needs of the cell are not being met because of a deficiency of oxaloacetate. Under these conditions, the pyruvate carboxylase catalyzes an anaplerotic reaction. Alternatively, acetyl CoA might accumulate because the energy needs of the cell have been met. In this circumstance, pyruvate will be converted back into glucose, and the first step in this conversion is the formation of oxaloacetate.
 CLINICAL INSIGHT
CLINICAL INSIGHTDefects in the Citric Acid Cycle Contribute to the Development of Cancer
Four enzymes crucial to cellular respiration are known to contribute to the development of cancer: succinate dehydrogenase, fumarase, pyruvate dehydrogenase kinase, and isocitrate dehydrogenase Mutations that alter the activity of the first three of these enzymes enhance aerobic glycolysis. In aerobic glycolysis, cancer cells preferentially metabolize glucose to lactate even in the presence of oxygen. Defects in all of these enzymes have a common biochemical link: the transcription factor hypoxia inducible factor 1 (HIF-
Normally, HIF-
Defects in the enzymes of the citric acid cycle can significantly alter the regulation of prolyl hydroxylase 2. When either succinate dehydrogenase or fumarase is defective, succinate and fumarate accumulate in the mitochondria and spill over into the cytoplasm. Both succinate and fumarate are competitive inhibitors of prolyl hydroxylase 2. The inhibition of prolyl hydroxylase 2 results in the stabilization of HIF-
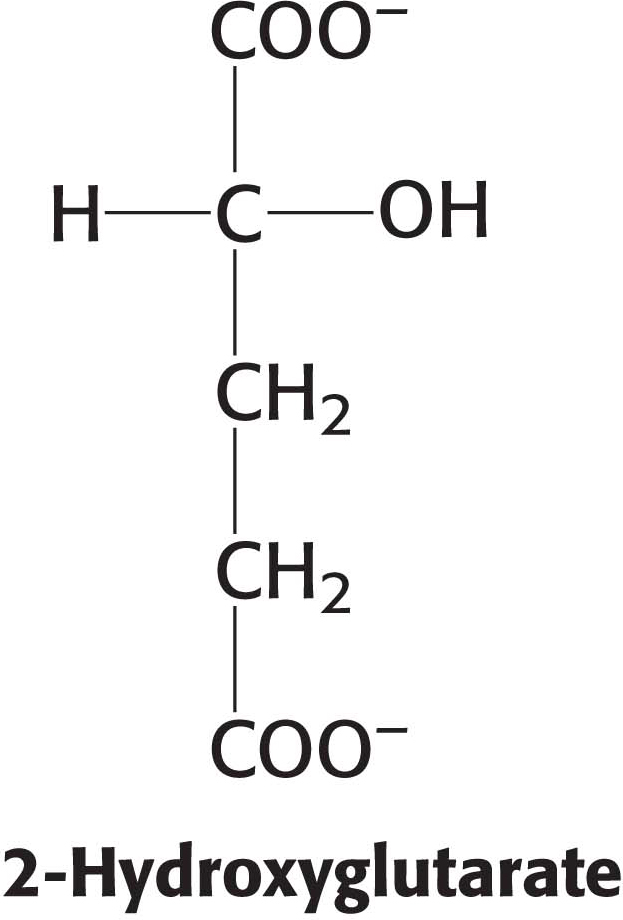
Mutations in isocitrate dehydrogenase result in the generation of an oncogenic metabolite, 2-
These observations linking citric acid cycle enzymes to cancer suggest that cancer is also a metabolic disease, not simply a disease of mutant growth factors and cell-