1.2 There Are Four Major Classes of Biomolecules
✓ 1 Describe the key classes of biomolecules and differentiate between them.
Living systems contain a dizzying array of biomolecules. However, these biomolecules can be divided into just four classes: proteins, nucleic acids, lipids, and carbohydrates.
Proteins Are Highly Versatile Biomolecules
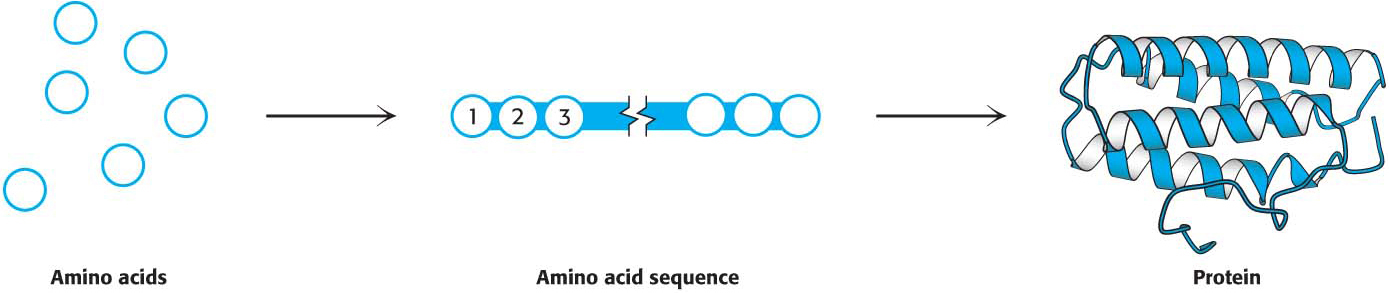
Much of our study of biochemistry will revolve around proteins. Proteins are constructed from 20 building blocks, called amino acids, linked by peptide bonds to form long unbranched polymers (Figure 1.1). These polymers fold into precise three-
Nucleic Acids Are the Information Molecules of the Cell
As information keepers of the cell, the primary function of nucleic acids is to store and transfer information. They contain the instructions for all cellular functions and interactions. Like proteins, nucleic acids are linear molecules. However, nucleic acids are constructed from only four building blocks called nucleotides. A nucleotide is made up of a five-
There are two types of nucleic acid: deoxyribonucleic acid (DNA) and ribonucleic acid (RNA). Genetic information is stored in DNA—
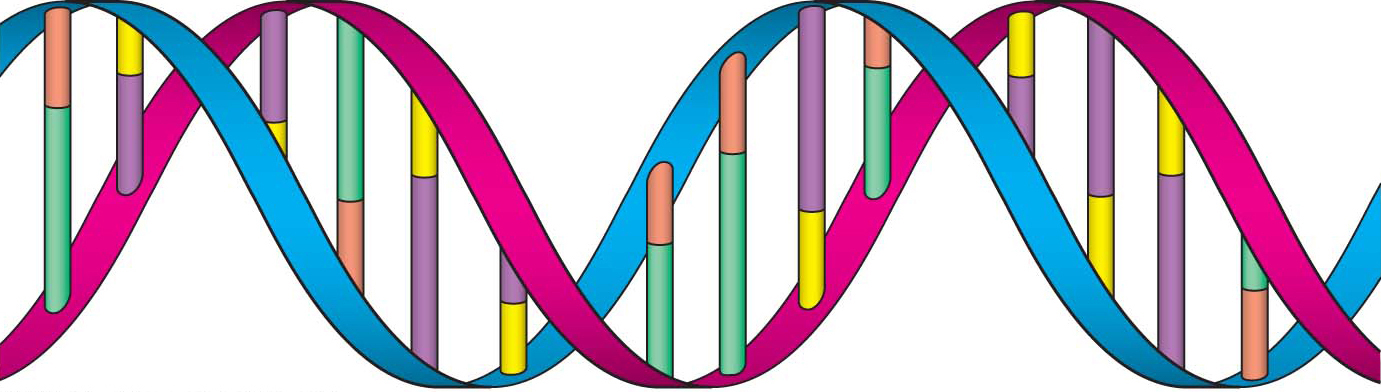
RNA is a single-
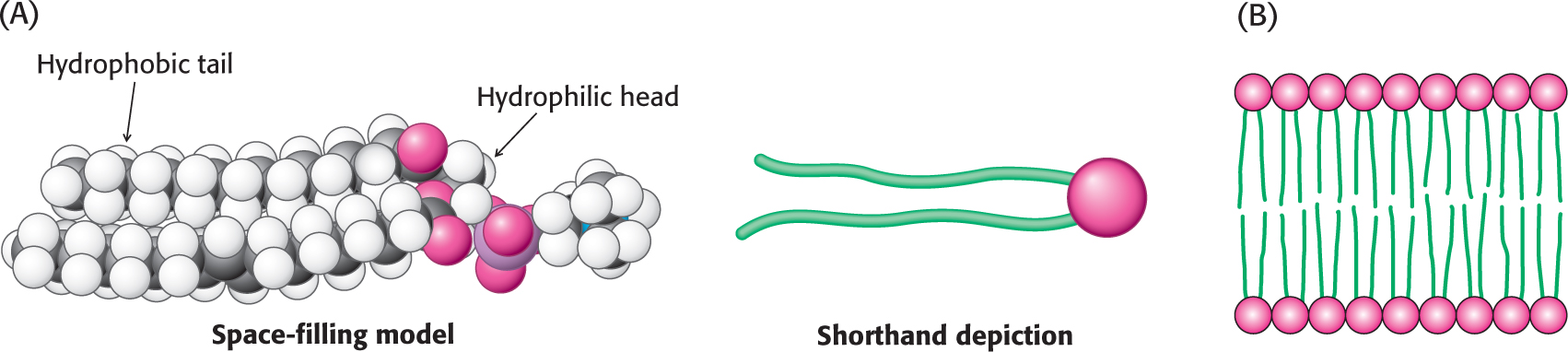
Lipids Are a Storage Form of Fuel and Serve as a Barrier
Among the key biomolecules, lipids are much smaller than proteins or nucleic acids. Whereas proteins and nucleic acids can have molecular weights of thousands to millions, a typical lipid has a molecular weight of 1300 g mol−1. Moreover, lipids are not polymers made of repeating units, as are proteins and nucleic acids. A key characteristic of many biochemically important lipids is their dual chemical nature: part of the molecule is hydrophilic, meaning that it can dissolve in water, whereas the other part, made up of one or more hydrocarbon chains, is hydrophobic and cannot dissolve in water (Figure 1.4). This dual nature allows lipids to form barriers that delineate the cell from its environment and to establish intracellular compartments. In other words, lipids allow the development of “inside” and “outside” at a biochemical level. The hydrocarbon chains cannot interact with water and, instead, interact with those of other lipids to form a barrier, or membrane, whereas the water-
Carbohydrates Are Fuels and Informational Molecules
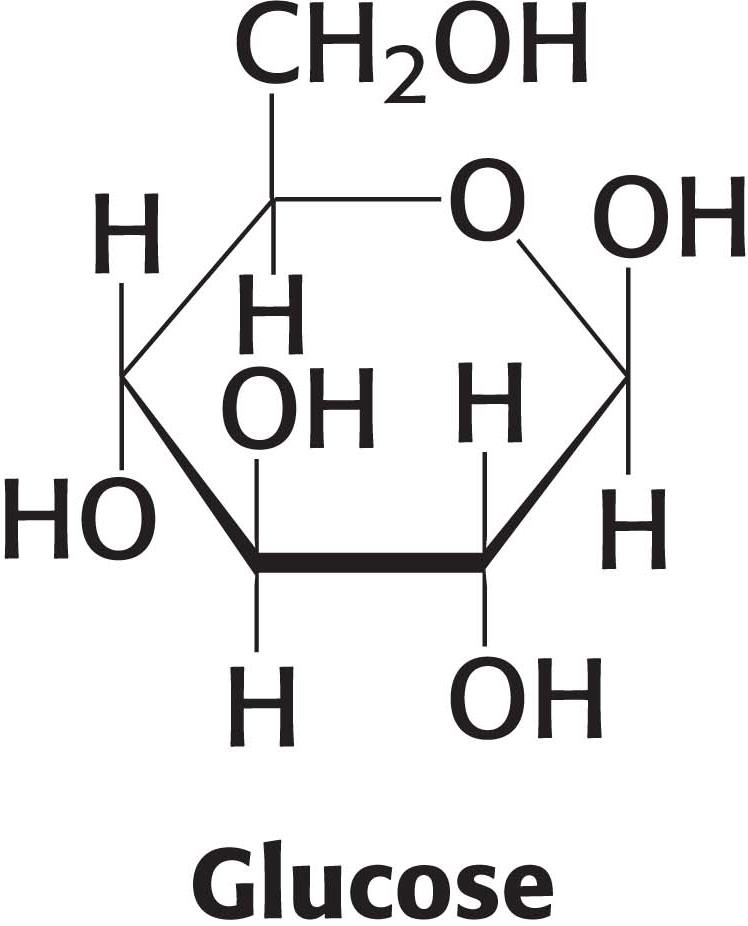
Most of us already know that carbohydrates are an important fuel source for most living creatures. The most-
QUICK QUIZ 1
Name the four classes of biomolecules, and state an important function of each class.
Proteins: catalysts. Nucleic acids: information transfer. Lipids: fuel and structure. Carbohydrates: fuel and cell-
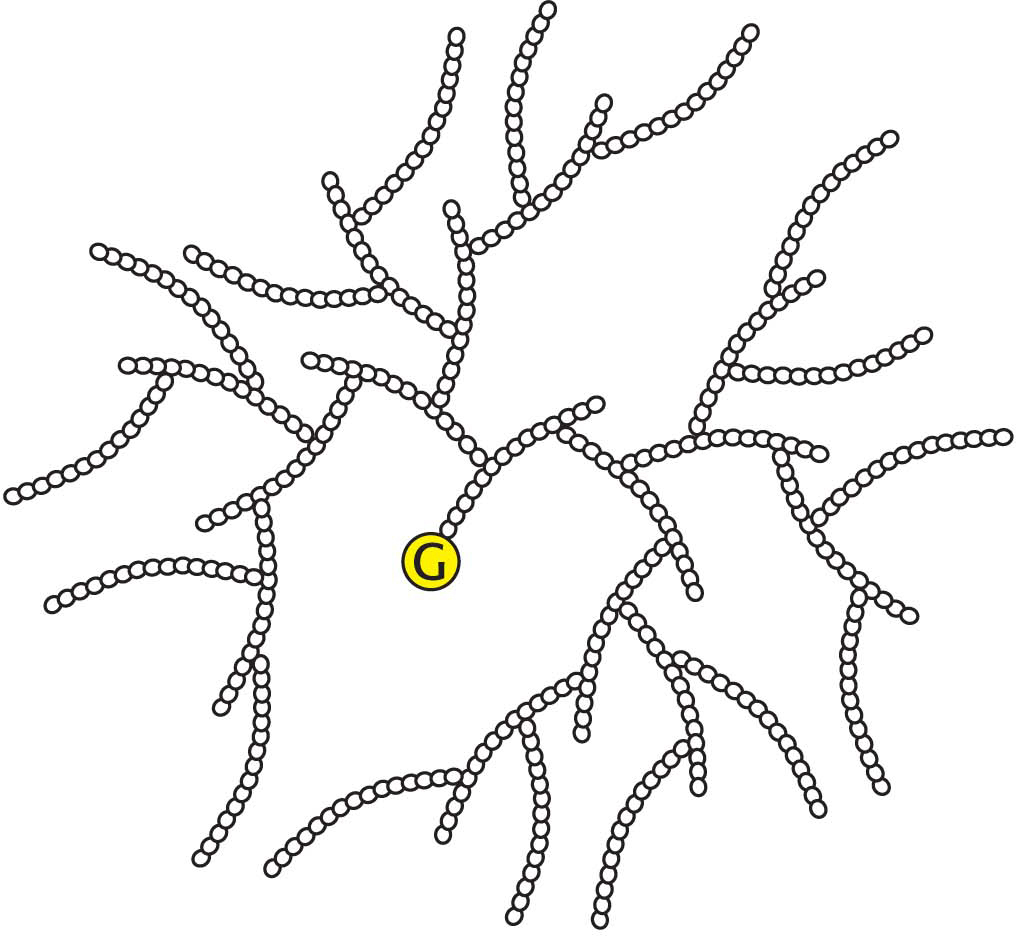
There are thousands of different carbohydrates. They can be linked together in chains, and these chains can be highly branched, much more so than in glycogen or starch. Such chains of carbohydrates play important roles in helping cells to recognize one another. Many of the components of the cell exterior are decorated with various carbohydrates that can be identified by other cells and serve as sites of cell-