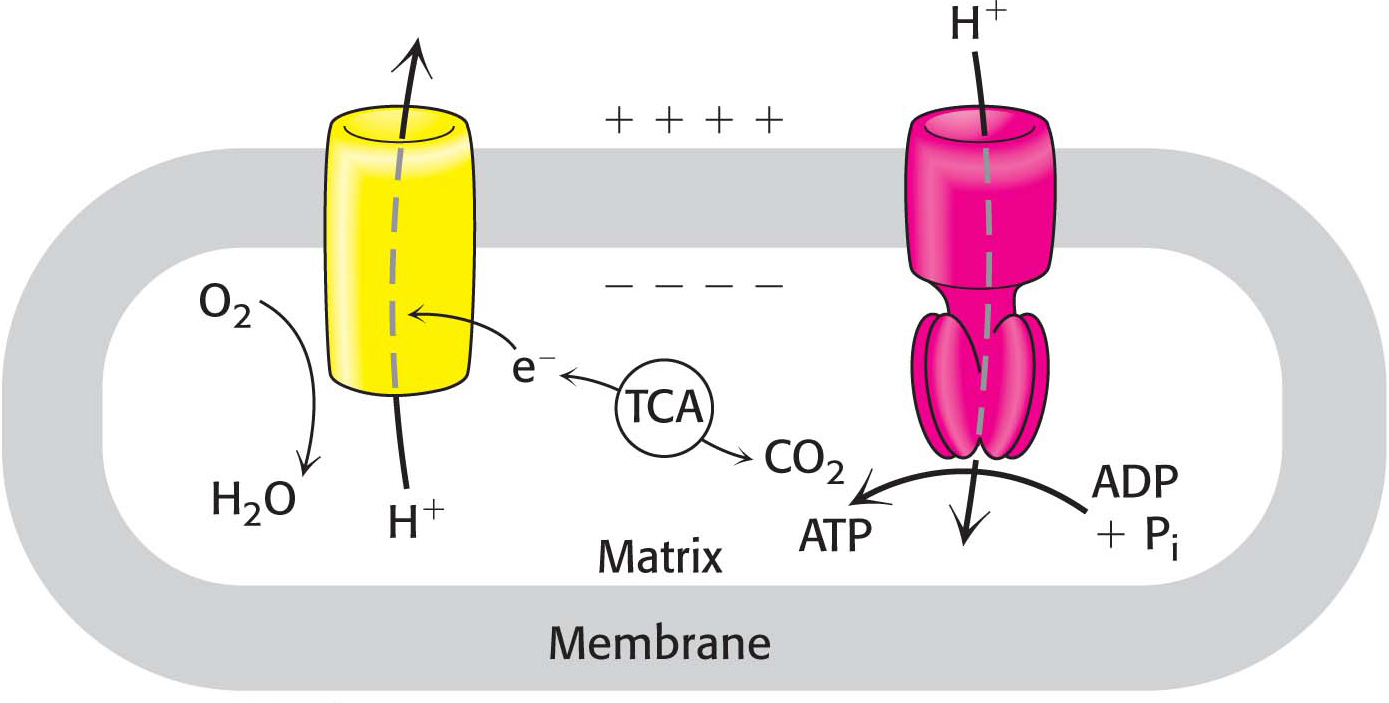
We begin our study of oxidative phosphorylation by examining the oxidation–reduction reactions that allow the flow of electrons from NADH and FADH2 to oxygen. The electron flow, which is very exergonic, takes place in four large protein complexes that are embedded in the inner mitochondrial membrane, together called the respiratory chain or the electron-transport chain. Importantly, three of these complexes use the energy released by the electron flow to pump protons from the mitochondrial matrix into the space between the inner and outer mitochondrial membranes. The proton gradient is then used to power the synthesis of ATP by oxidative phosphorylation, a process that we will examine in Chapter 21 (Figure 20.1). Collectively, the generation of high-transfer-potential electrons by the citric acid cycle, their flow through the respiratory chain, and the accompanying synthesis of ATP is called respiration or cellular respiration.