The Electron-Transfer Potential of an Electron Is Measured as Redox Potential
In oxidative phosphorylation, the electron-transfer potential of NADH or FADH2 is converted into the phosphoryl-transfer potential of ATP. To better understand this conversion, we need quantitative expressions for these forms of free energy. The measure of phosphoryl-transfer potential is already familiar to us: it is given by ΔG°′ for the hydrolysis of the phosphoryl compound. The corresponding expression for the electron-transfer potential is E′0, the reduction potential (also called the redox potential or oxidation–reduction potential).
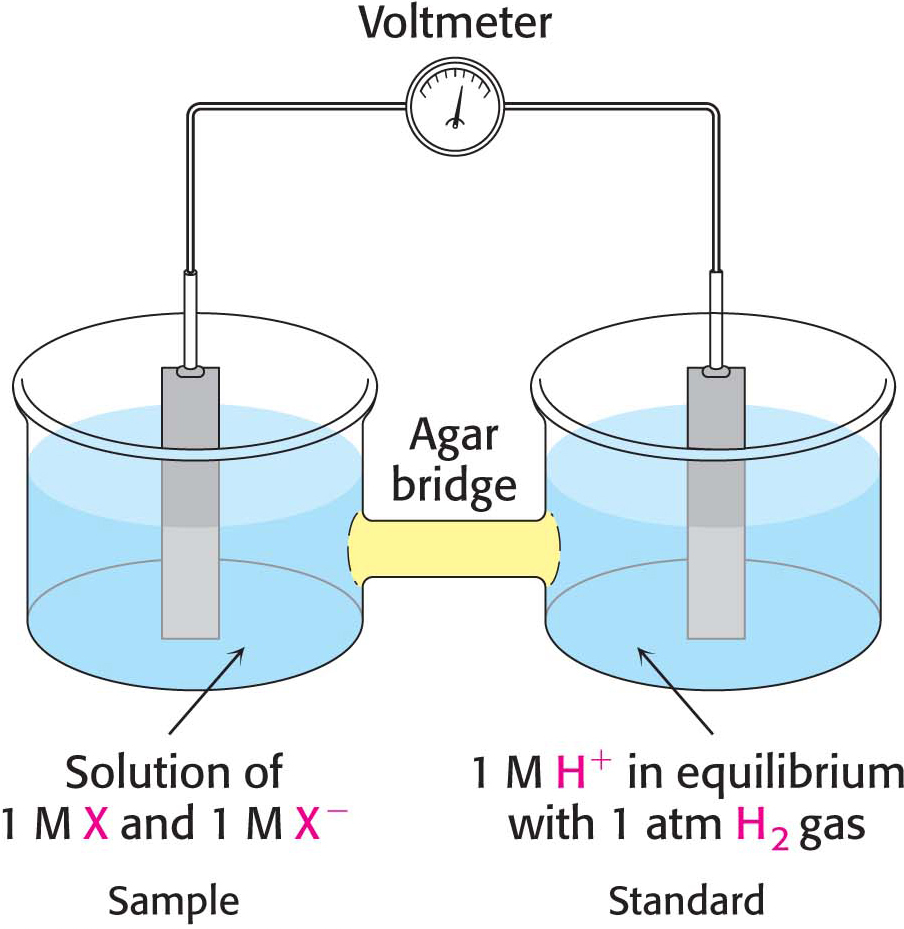
Consider a substance that can exist in an oxidized form, X, and a reduced form, X−. Such a pair is called a redox couple. The reduction potential of this couple can be determined by measuring the electromotive force generated by a sample half-cell connected to a standard reference half-cell (Figure 20.4). The sample half-cell consists of an electrode immersed in a solution of 1 M oxidant (X) and 1 M reductant (X−). The standard reference half-cell consists of an electrode immersed in a 1 M H+ solution that is in equilibrium with H2 gas at 1 atmosphere (1 atm) of pressure. The electrodes are connected to a voltmeter, and an agar (a gelatinous polysaccharide) bridge allows the flow of ions between the half-cells. Electrons then flow from one half-cell to the other through the wire connecting the two cells. If the reaction proceeds in the direction
the reactions in the half-cells (referred to as half-reactions or couples) must be
Thus, electrons flow from the sample half-cell to the standard reference half-cell, and the sample-cell electrode is taken to be negative with respect to the standard-cell electrode. The reduction potential of the X:X– couple is the observed voltage at the start of the experiment (when X, X−, and H+ are 1 M with 1 atm of H2). The reduction potential of the H+:H2 couple is defined to be 0 volts.
DID YOU KNOW?
An oxidant (oxidizing agent) is the acceptor of electrons in an oxidation–reduction reaction.
A reductant (reducing agent) is the donator of electrons in an oxidation–reduction reaction.
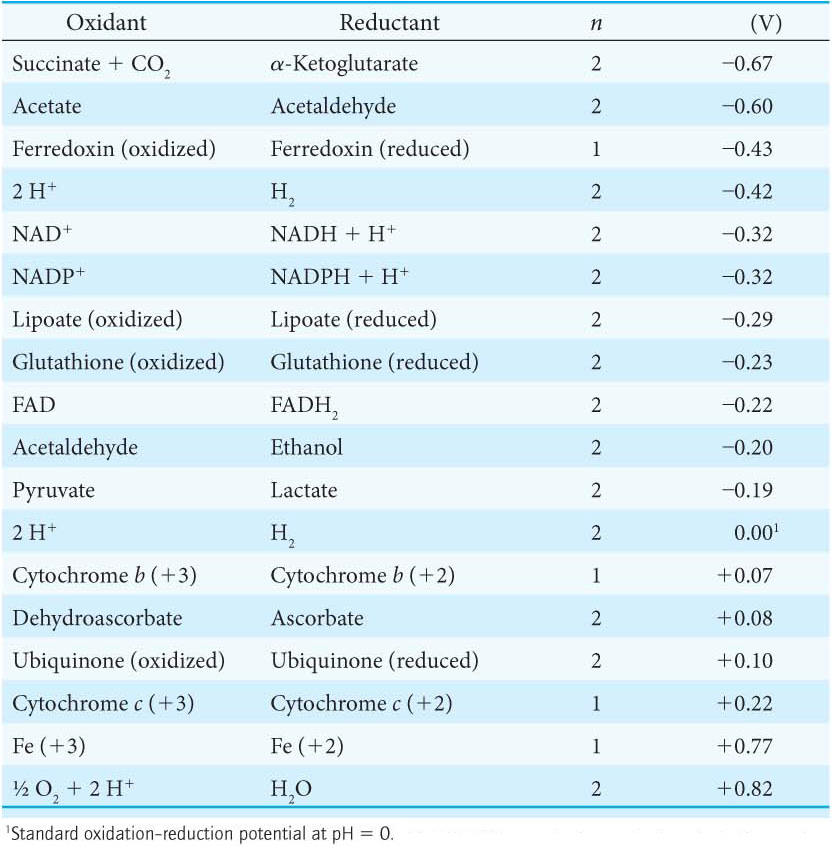
The meaning of the reduction potential is now evident. A negative reduction potential means that the oxidized form of a substance has lower affinity for electrons than does H2, as in the preceding example. A positive reduction potential means that the oxidized form of a substance has higher affinity for electrons than does H2. These comparisons refer to standard conditions—namely, 1 M oxidant, 1 M reductant, 1 M H+, and 1 atm H2. Thus, a strong reducing agent (such as NADH) is poised to donate electrons and has a negative reduction potential, whereas a strong oxidizing agent (such as O2) is ready to accept electrons and has a positive reduction potential. The reduction potentials of many biologically important redox couples are known (Table 20.1). Table 20.1 is like those presented in chemistry textbooks, except that a hydrogen ion concentration of 10–7 M (pH 7) instead of 1 M (pH 0) is the standard state adopted by biochemists. This difference is denoted by the prime in E′0. Recall that the prime in ΔG°′ denotes a standard free-energy change at pH 7.
Page 367
The standard free-energy change ΔG°′ is related to the change in reduction potential ΔE′0
in which n is the number of electrons transferred, F is a proportionality constant called the faraday (96.48 kJ mol–1 V–1, or 23.06 kcal mol–1 V–1), ΔE′0 is in volts, and ΔG°′ is in kilojoules or kilocalories per mole.
Electron Flow Through the Electron-Transport Chain Creates a Proton gradient
The driving force of oxidative phosphorylation is the electron-transfer potential of NADH or FADH2 relative to that of O2. Recall that NADH and FADH2 are carriers of high-transfer-potential electrons generated in the citric acid cycle and elsewhere in metabolism. How much energy is released by the reduction of O2 with NADH? Let us calculate ΔG°′ for this reaction. The pertinent half-reactions are
Page 368
Subtracting reaction B from reaction A yields
Note that the sign of E’0 for the NAD+/NADH half-reaction is changed when NADH donates electrons. The standard free energy for this reaction is then given by the following equation:
Substituting the appropriate values yields
This release of free energy is substantial. Recall that ΔG°′ for the synthesis of ATP is +30.5 kJ mol–1 (+7.3 kcal mol–1). The energy released by the reduction of each electron carrier generates a proton gradient that is then used for the synthesis of ATP and the transport of metabolites across the mitochondrial membrane. Indeed, each proton transported out of the matrix to the cytoplasmic side corresponds to 21.8 kJ mol–1 (5.2 kcal mol–1) of free energy.
The Electron-Transport Chain Is a series of Coupled Oxidation–Reduction Reactions
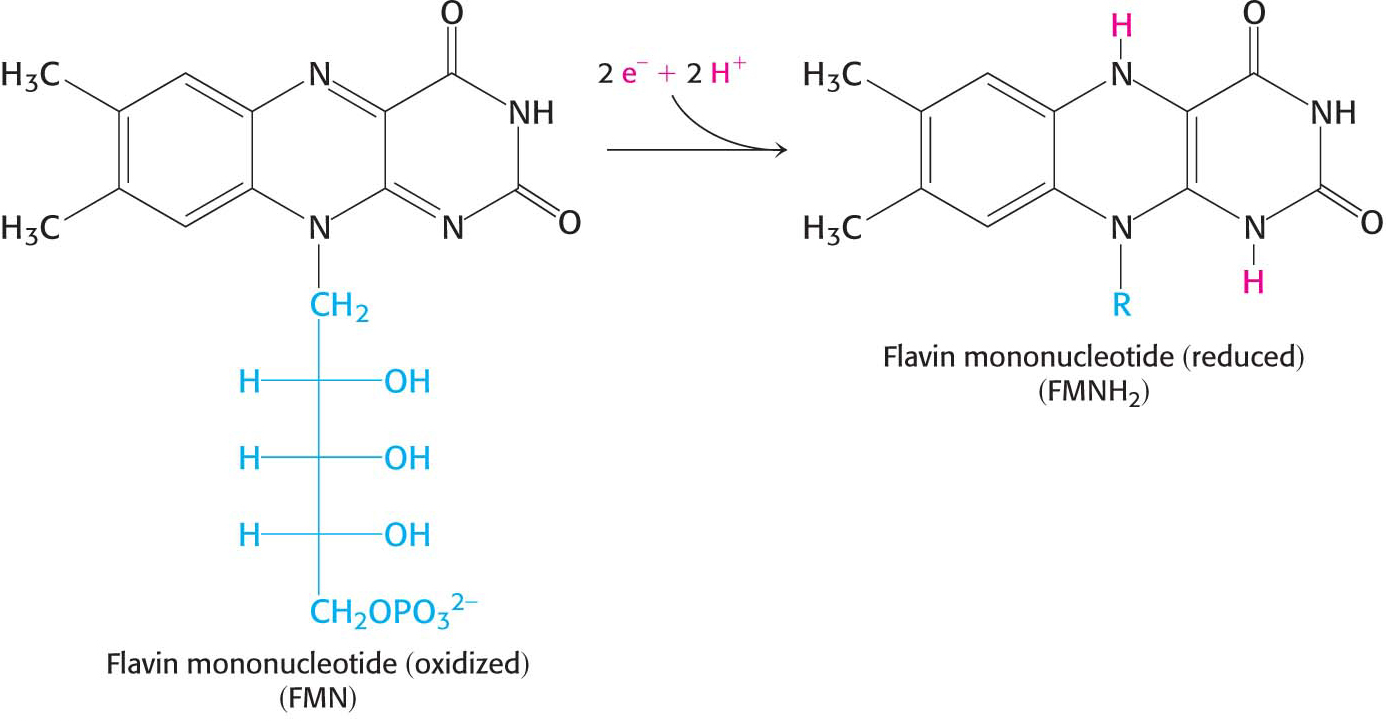
Electron flow from NADH to O2 is accomplished by a series of intermediate electron carriers—a bucket brigade of electron carriers—that are coupled as members of sequential redox reactions. As we will see, NADH is oxidized by passing electrons to flavin mononucleotide (FMN), an electron carrier similar to flavin adenine dinucleotide (FAD) but lacking the nucleotide component (Figure 20.5). Reduced FMN is subsequently oxidized by the next electron carrier in the chain, and the process repeats itself as the electrons flow down the electron-transport chain until they finally reduce O2. The members of the electron-transport chain are arranged so that the electrons always flow to components with more positive reduction potentials (a higher electron affinity).
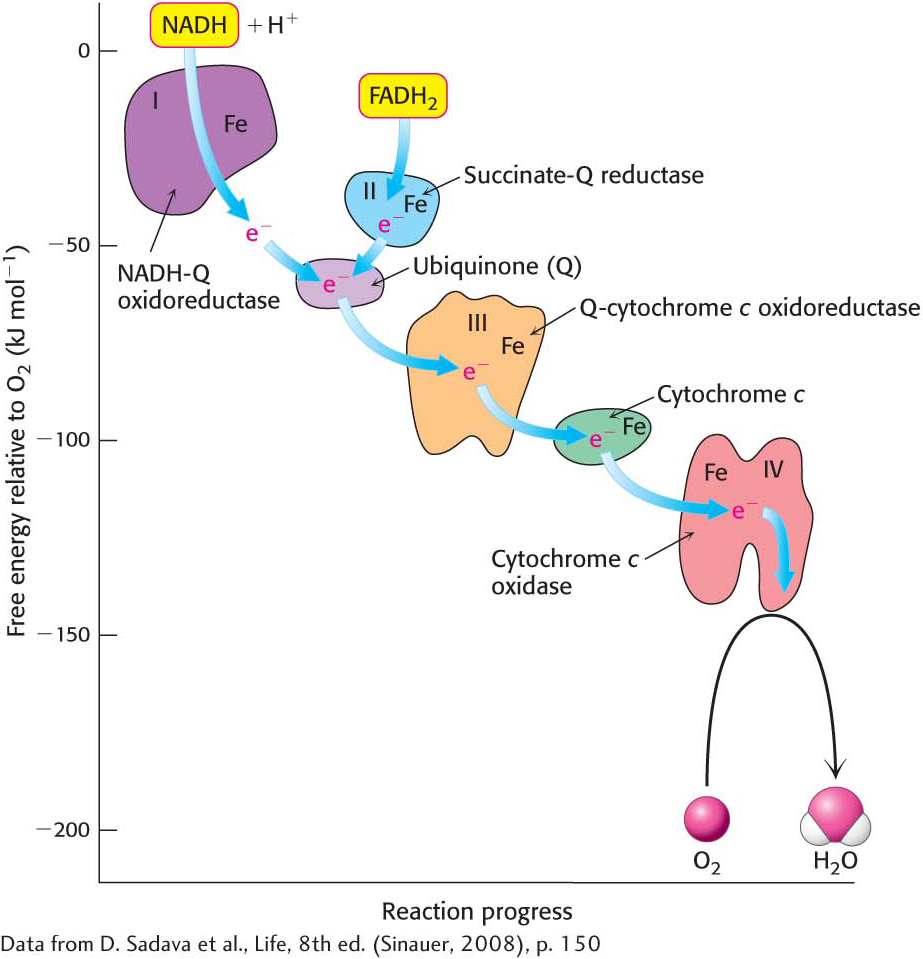
Electrons are transferred from NADH to O2 through a chain of three large protein complexes called NADH-Q oxidoreductase, Q-cytochrome c oxidoreductase, and cytochrome c oxidase (Figure 20.6). These complexes appear to be associated in a supramolecular complex termed the respirasome. As in the citric acid cycle, such supramolecular complexes facilitate the rapid transfer of substrate and prevent the release of reaction intermediates. A fourth large protein complex, called succinate-Q reductase, contains the succinate dehydrogenase that generates FADH2 in the citric acid cycle. Electrons from this FADH2 enter the electron-transport chain at Q-cytochrome c oxidoreductase.
Page 369
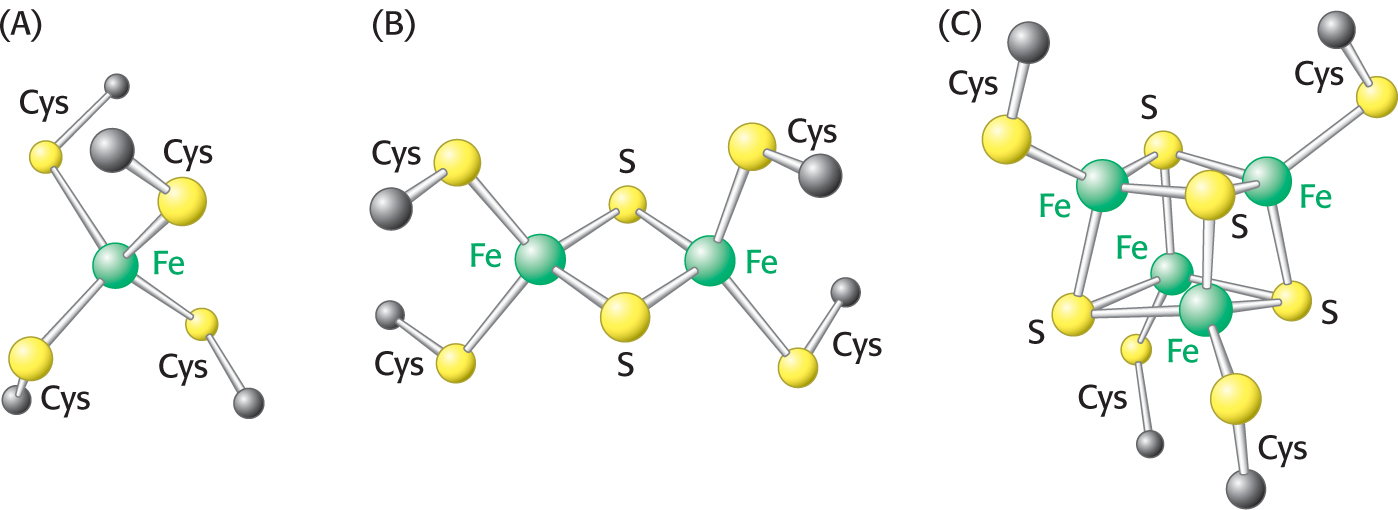
Before we look at how NADH and FADH2 reduce O2 in more detail, it’s worth pointing out several features of the electron-transport chain that affect the energy yield of the high-transfer-potential electrons and facilitate the efficient flow of electrons (Figure 20.6). First of all, electrons from FADH2 feed into the chain “downstream” of those from NADH because the electrons of FADH2 have a lower reduction potential (Table 20.1). As a result, FADH2-derived electrons pump fewer protons and thus yield fewer molecules of ATP. Second, note that iron is a prominent electron carrier, appearing in several places. Iron in the electron-transport chain appears in two fundamental forms: associated with sulfur as iron–sulfur clusters located in iron–sulfur proteins (also called nonheme-iron proteins; Figure 20.7), and as components of a heme-prosthetic group, which are embedded in a special class of proteins called cytochromes (Figure 20.8). In both iron–sulfur proteins and cytochromes, iron shuttles between its reduced ferrous (Fe2+) and its oxidized ferric state (Fe3+):
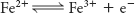
Page 370
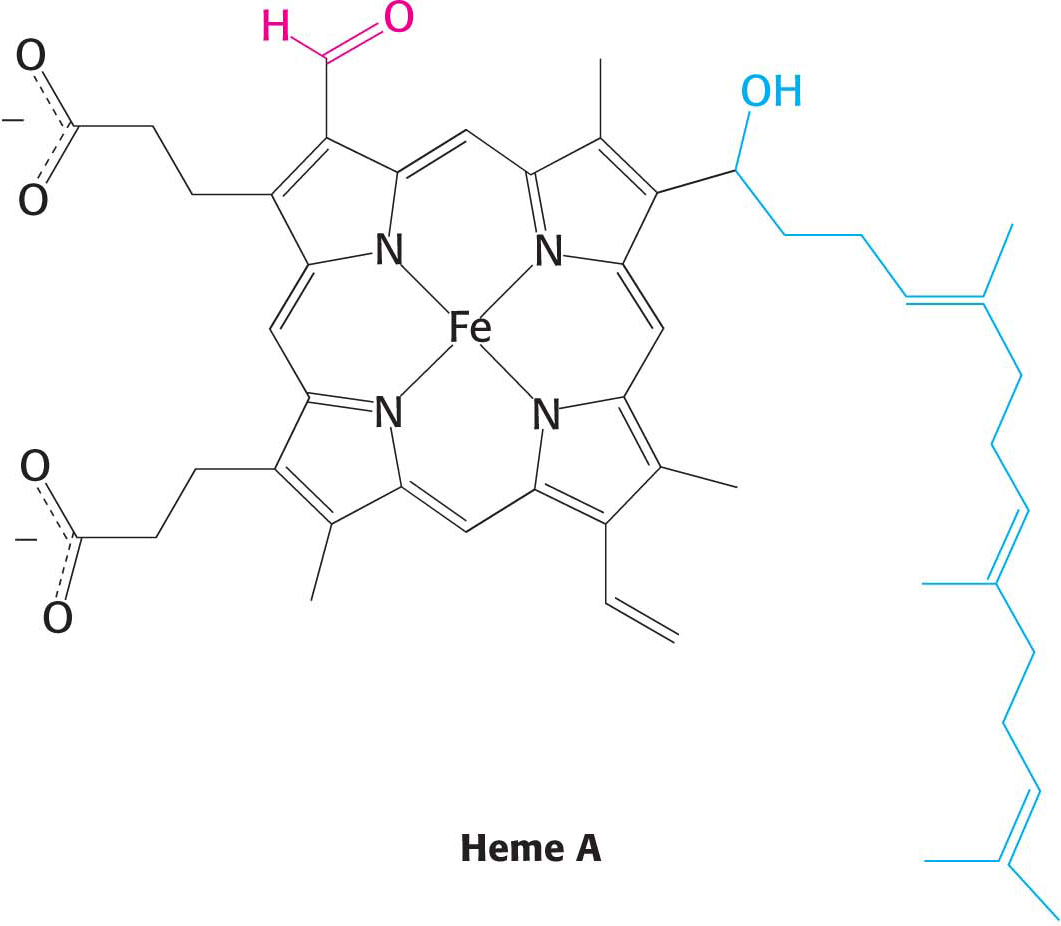
The heme-prosthetic group of cytochromes is iron-protoporphyrin IX, the same heme present in hemoglobin and myoglobin. The iron in hemoglobin and myoglobin, in contrast to the iron in cytochromes, remains in the Fe2+ oxidation state.
The fact that iron is an electron carrier in several places in the electron-transport chain raises a puzzling question. If the flow of electrons from NADH to O2 is exergonic and iron has a reduction potential of +0.77 V (Table 20.1), how can iron participate in several places in the electron-transport chain if each step is exergonic? In other words, how can iron have several different reduction potentials? The answer to the puzzle is that the oxidation–reduction potential of iron ions can be altered by their environment. In regard to the electron-transport chain, the iron ion is not free; rather, it is embedded in different proteins, enabling iron to have various reduction potentials and to play a role at several different locations in the chain.
The metal copper also appears as a member of the final component of the electron-transport chain, where it is alternatively oxidized and reduced:
Another key feature of the electron-transport chain is the prominence of coenzyme Q (Q) as an electron carrier. Coenzyme Q, also known as ubiquinone because it is a ubiquitous quinone in biological systems, is a quinone derivative with a long isoprenoid tail, which renders the molecule hydrophobic and allows it to diffuse rapidly within the inner mitochondrial membrane, where it shuttles protons and electrons about.
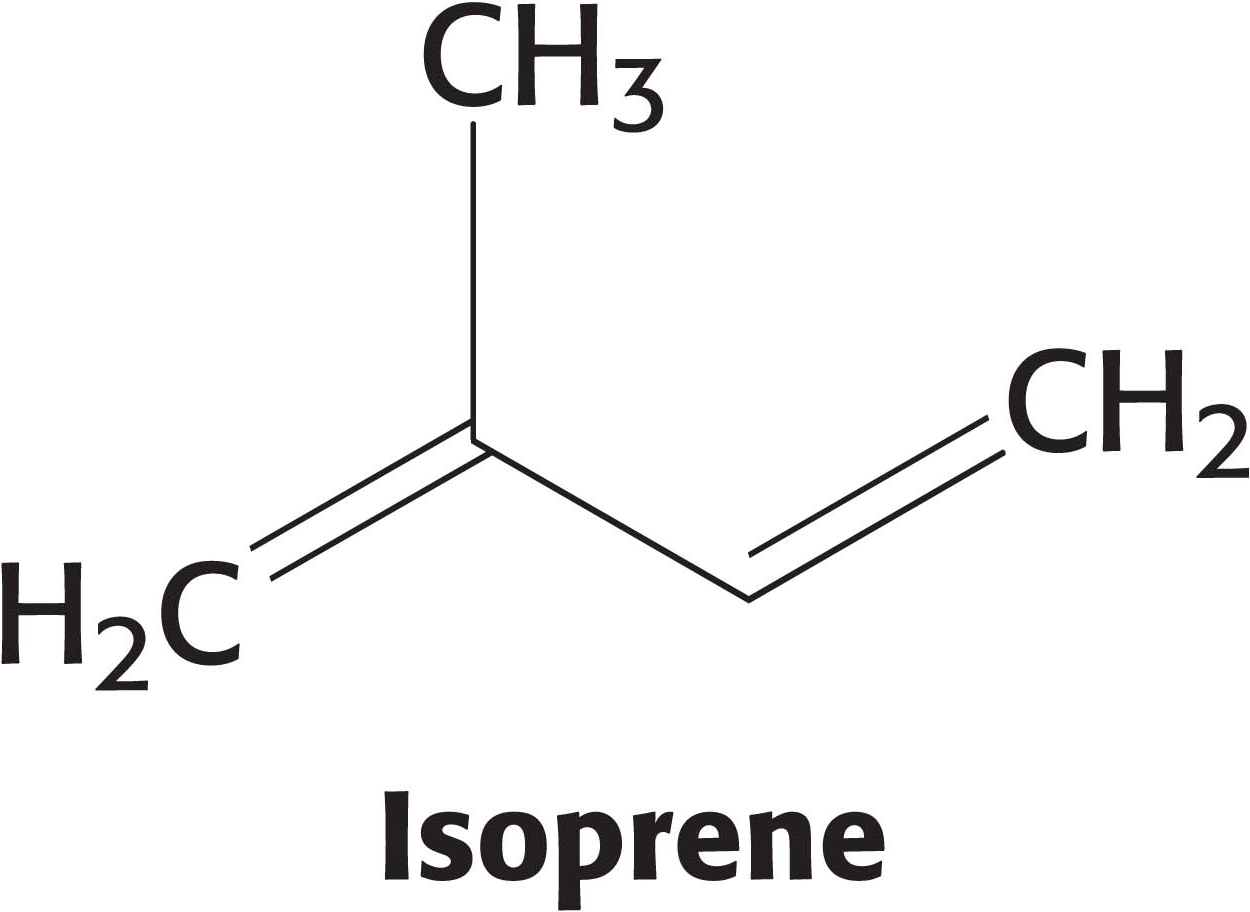
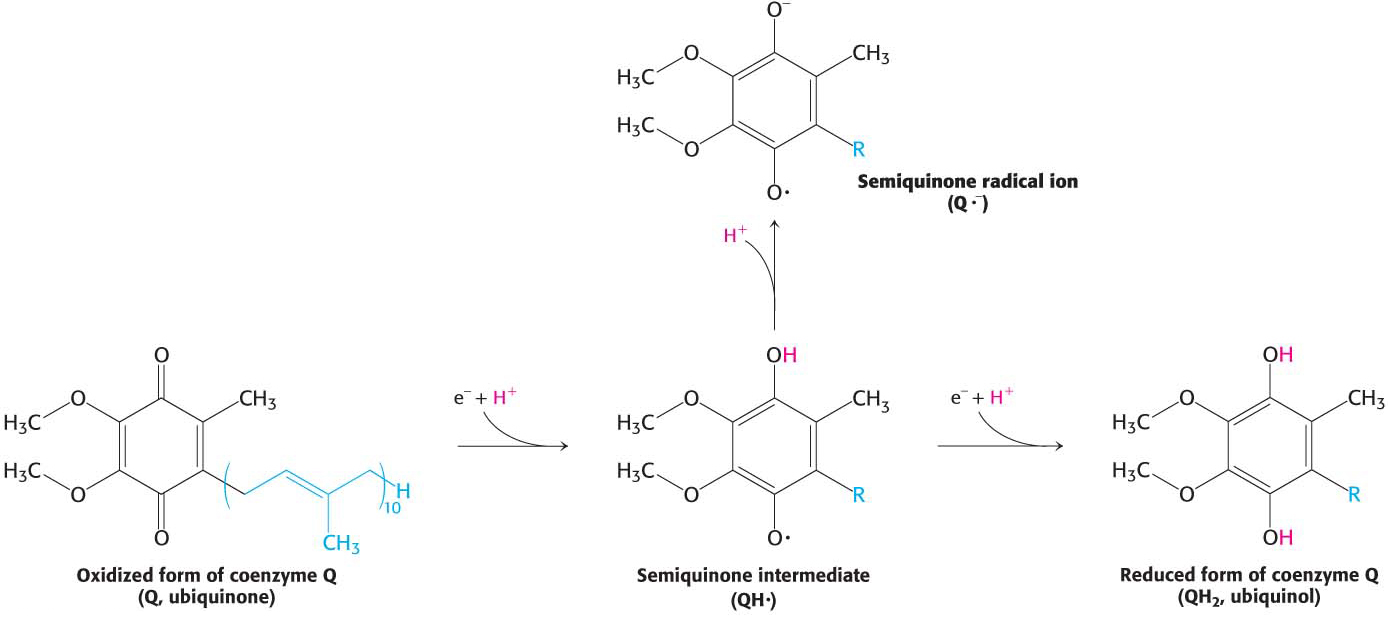
Coenzyme Q consists of more than one five-carbon isoprene unit. The exact number depends on the species in which it is found. The most common form in mammals contains 10 isoprene units (coenzyme Q10). For simplicity, the subscript will be omitted from this abbreviation because all varieties function in an identical manner. Quinones can exist in several oxidation states (Figure 20.9). In the fully oxidized state (Q), coenzyme Q has two keto groups. The addition of one electron yields a semiquinone radical anion (Q∙−), whereas the addition of one electron and one proton results in the semiquinone form (QH∙). The addition of a second electron and proton generates ubiquinol (QH2), the fully reduced form of coenzyme Q. Thus, for quinones, electron-transfer reactions are coupled to proton binding and release, a property that is key to transmembrane proton transport. A pool of Q and QH2—the Q pool—is thought to exist in the inner mitochondrial membrane, although recent research suggests that it is confined to the respirasome.
Page 371
 CLINICAL INSIGHT
CLINICAL INSIGHTLoss of Iron-Sulfur Cluster Results in Friedreich’s Ataxia
The importance of Fe-S clusters is illustrated by the loss of function of the protein frataxin. Frataxin is a small (14.2 kDa) mitochondrial protein that is crucial for the synthesis of Fe-S clusters. Mutations in frataxin causes Friedreich’s ataxia, a disease of varying severity that affects the central and peripheral nervous systems as well as the heart and skeletal systems. Severe cases lead to death in young adult life. The most common mutation is trinucleotide expansion (Section 35.1) in the gene for frataxin.


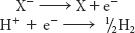
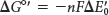

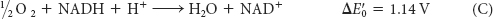
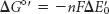
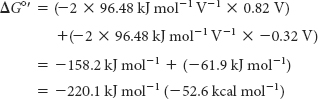
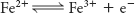
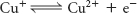
 CLINICAL INSIGHT
CLINICAL INSIGHT