
20.3 The Respiratory Chain Consists of Proton Pumps and a Physical Link to the Citric Acid Cycle
✓ 2 Explain the benefits of having the electron-
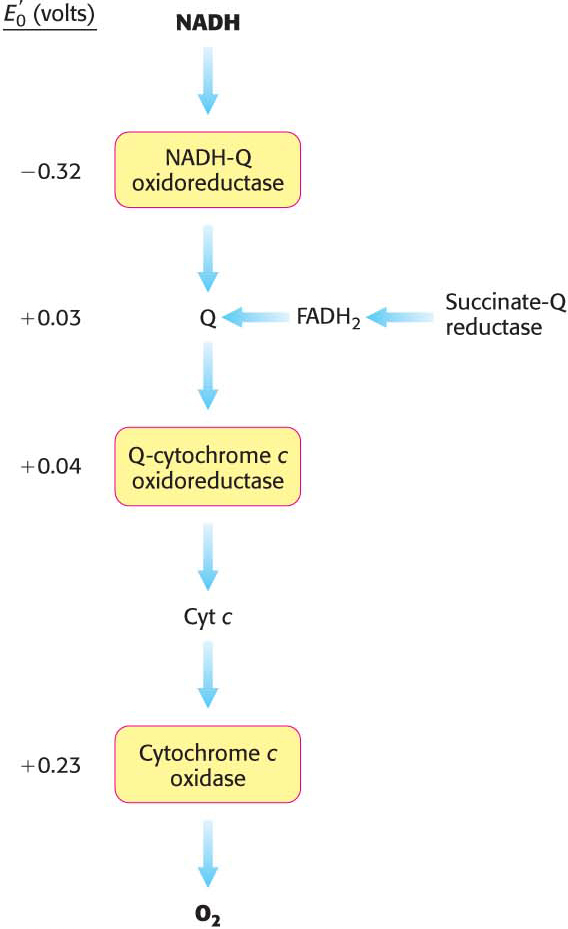
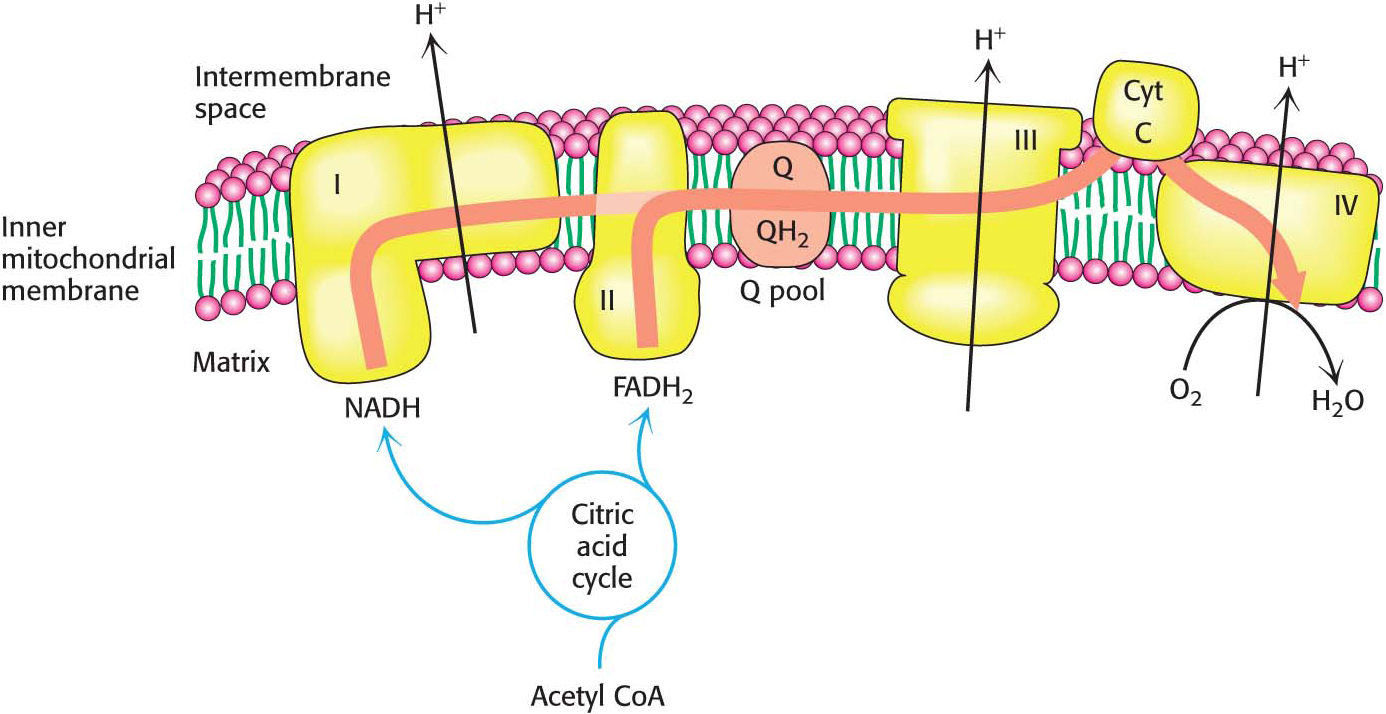
How are the various electron carriers arranged to yield an exergonic reaction that ultimately results in the generation of a proton gradient? As discussed previously (Figure 20.6), electrons are transferred from NADH to O2 through a chain of three protein complexes (Table 20.2 and Figure 20.10). Electron flow within these transmembrane complexes leads to the transport of protons across the inner mitochondrial membrane. A fourth protein complex, succinate-
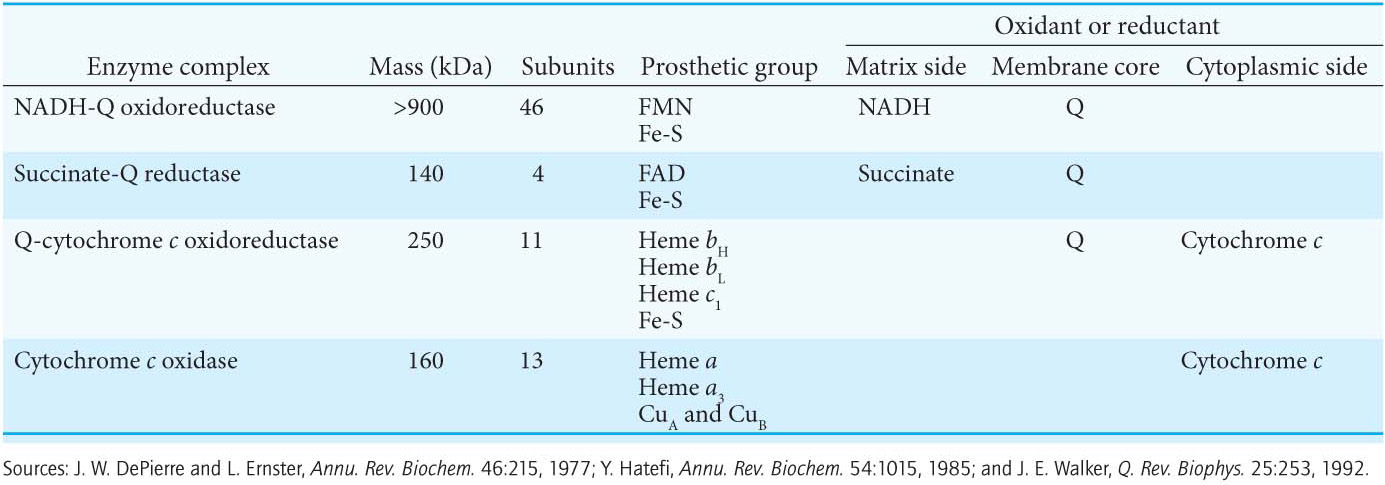
The High+-Potential Electrons of NADH Enter the Respiratory Chain at NADH-Q Oxidoreductase
The electrons of NADH enter the chain at NADH-
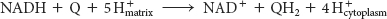
Recent structural studies have suggested how Complex I acts as a proton pump. What are the structural elements required for proton pumping? The membrane-
NUTRITION FACT
Coenzyme Q is frequently marketed as a dietary supplement, often called CoQ 10. Its proponents assert that it boosts energy, enhances the immune system, and acts as an antioxidant. Clearly, people who lack the ability to synthesize CoQ and consequently suffer from neuromuscular disorders due to defective mitochondria benefit from CoQ supplementation. Moreover, some evidence is beginning to accumulate that shows that CoQ supplementation may benefit people undergoing severe physiological stress—
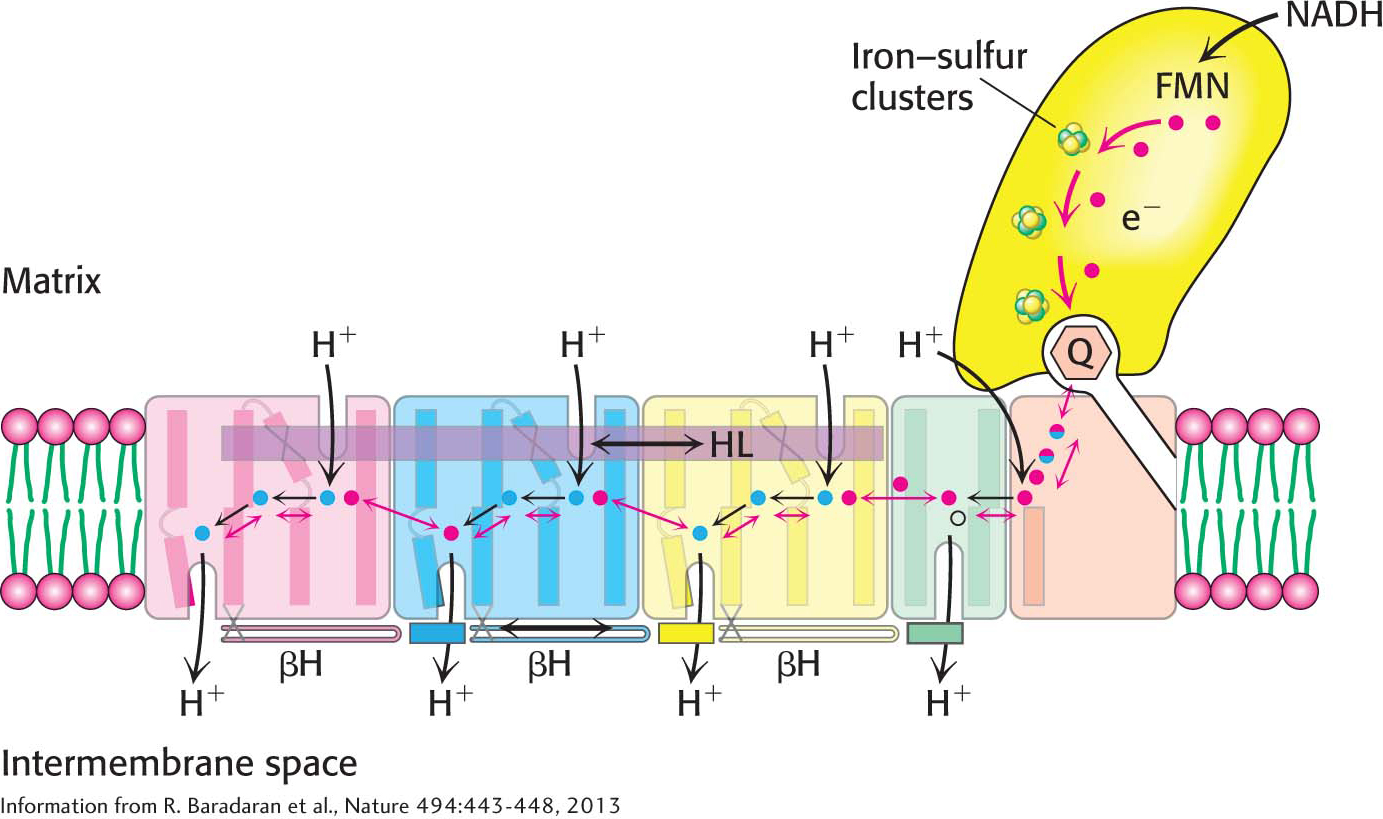
How do these structural elements cooperate to pump protons out of the matrix? When Q accepts two electrons from NADH, generating Q2−,the negative charges on Q2− interact electrostatically with negatively charged amino acid residues in the membrane-
Ubiquinol Is the Entry Point for Electrons from FADH2 of Flavoproteins
Recall that FADH2 is formed in the citric acid cycle in the oxidation of succinate to fumarate by succinate dehydrogenase. This enzyme is part of the succinate-
Electrons Flow from Ubiquinol to Cytochrome c Through Q-Cytochrome c Oxidoreductase
The second of the three proton pumps in the respiratory chain is Q-
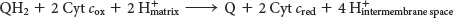
Q-
The Q Cycle Funnels Electrons from a Two-Electron Carrier to a One-Electron Carrier and Pumps Protons
QH2 passes two electrons to Q-
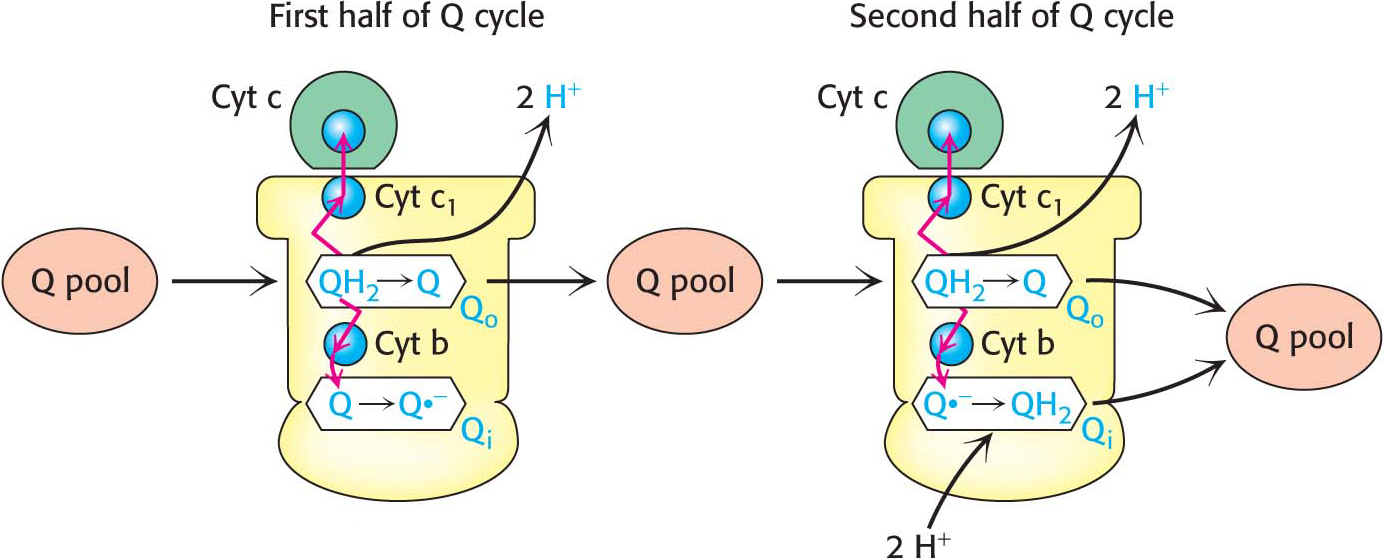
The cycle begins when two QH2 molecules bind to the complex consecutively, each giving up two electrons and two H+. These protons are released to the intermembrane space. QH2 binds to the first Q binding site (Qo), and the two electrons travel through the complex to different destinations. One electron flows, first, to the Rieske 2Fe-
The second electron passes through two heme groups of cytochrome b to an oxidized ubiquinone in a second Q binding site (Qi). The Q in the second binding site is reduced to a semiquinone radical anion (Q?−) by the electron from the first QH2. The now fully oxidized Q leaves the first Q site, free to reenter the Q pool.
A second molecule of QH2 binds to Q-
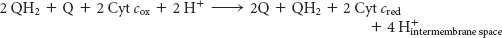
In one Q cycle, two QH2 molecules are oxidized to form two Q molecules, and then one Q molecule is reduced to QH2. The problem of how to efficiently funnel electrons from a two-
Cytochrome c Oxidase Catalyzes the Reduction of Molecular Oxygen to Water
The last of the three proton-
QUICK QUIZ 1
Why are the electrons carried by FADH2 not as energy rich as those carried by NADH? What is the consequence of this difference?
The reduction potential of FADH2 is less than that of NADH (Table 20.1). As a result, when those electrons are passed along to oxygen, less energy is released. The consequence of the difference is that electron flow from FADH2 to O2 pumps fewer protons than does the electron flow from NADH.
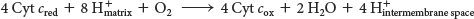
The requirement of oxygen for this reaction is what makes “aerobic” organisms aerobic. Obtaining oxygen for this reaction is the primary reason that human beings must breathe. Four electrons are funneled to O2 to completely reduce it to two molecules of H2O, and, concurrently, protons are pumped from the matrix to the cytoplasmic side of the inner mitochondrial membrane. This reaction is quite thermodynamically favorable. From the reduction potentials in Table 20.1, the standard free-
Cytochrome c oxidase, which consists of 13 subunits, contains two heme groups (heme a and heme a3) and three copper ions, arranged as two copper centers (CuA and CuB), with CuA containing two copper ions. Four molecules of reduced cytochrome c generated by Q-
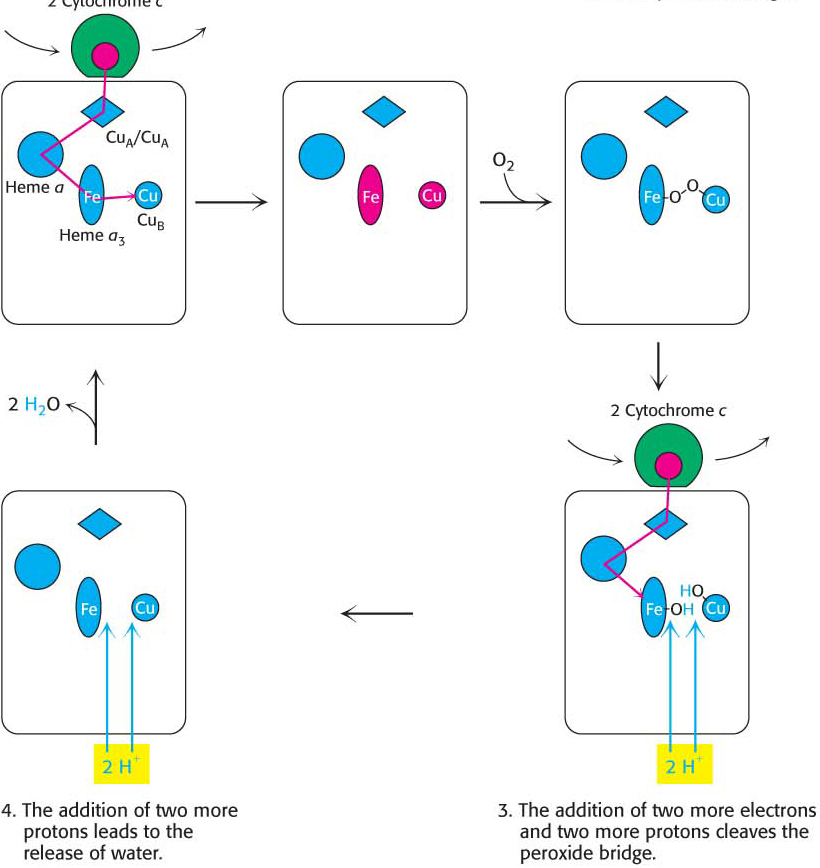
Electrons from two molecules of reduced cytochrome c flow through the oxidation–
reduction reactions, one stopping at CuB and the other at heme a3. With both centers in the reduced state, they together can now bind an oxygen molecule. As molecular oxygen binds, it removes an electron from each of the nearby ions in the active center to form a peroxide (O22−) bridge between them.
Two more molecules of cytochrome c bind and release electrons that travel to the active center. The addition of an electron as well as H+ to each oxygen atom reduces the two ion–
oxygen groups to CuB2+—OH and Fe3+—OH. Reaction with two more H+ ions allows the release of two molecules of H2O and resets the enzyme to its initial, fully oxidized form:
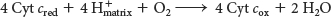
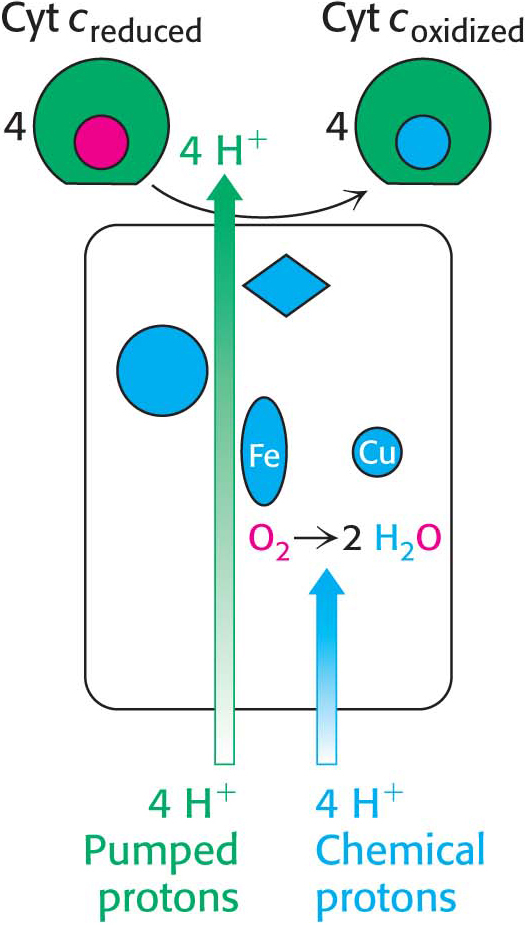
The four protons in this reaction come exclusively from the matrix. Thus, the consumption of these four protons contributes directly to the proton gradient. Consuming these four protons requires 87.2 kJ mol–1 (19.8 kcal mol–1), an amount substantially less than the free energy released from the reduction of oxygen to water. What is the fate of this missing energy? Remarkably, cytochrome c oxidase uses this energy to pump four additional protons from the matrix to the cytoplasmic side of the membrane in the course of each reaction cycle for a total of eight protons removed from the matrix (Figure 20.14). The details of how these protons are transported through the protein is still under study. Thus, the overall process catalyzed by cytochrome c oxidase is
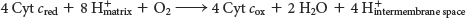
Figure 20.15 summarizes the flow of electrons from NADH and FADH2 through the respiratory chain. This series of exergonic reactions is coupled to the pumping of protons from the matrix. As we will see in Chapter 21, the energy inherent in the proton gradient will be used to synthesize ATP.
QUICK QUIZ 2
Amytal is a barbiturate sedative that inhibits electron flow through Complex I. How would the addition of amytal to actively respiring mitochondria affect the relative oxidation–
Complex I would be reduced, whereas Complexes II, III, and IV would be oxidized. The citric acid cycle would become reduced because it has no way to oxidize NADH.
DID YOU KNOW?
In anaerobic respiration in some organisms, chemicals other than oxygen are used as the final electron acceptor in an electron-
 BIOLOGICAL INSIGHT
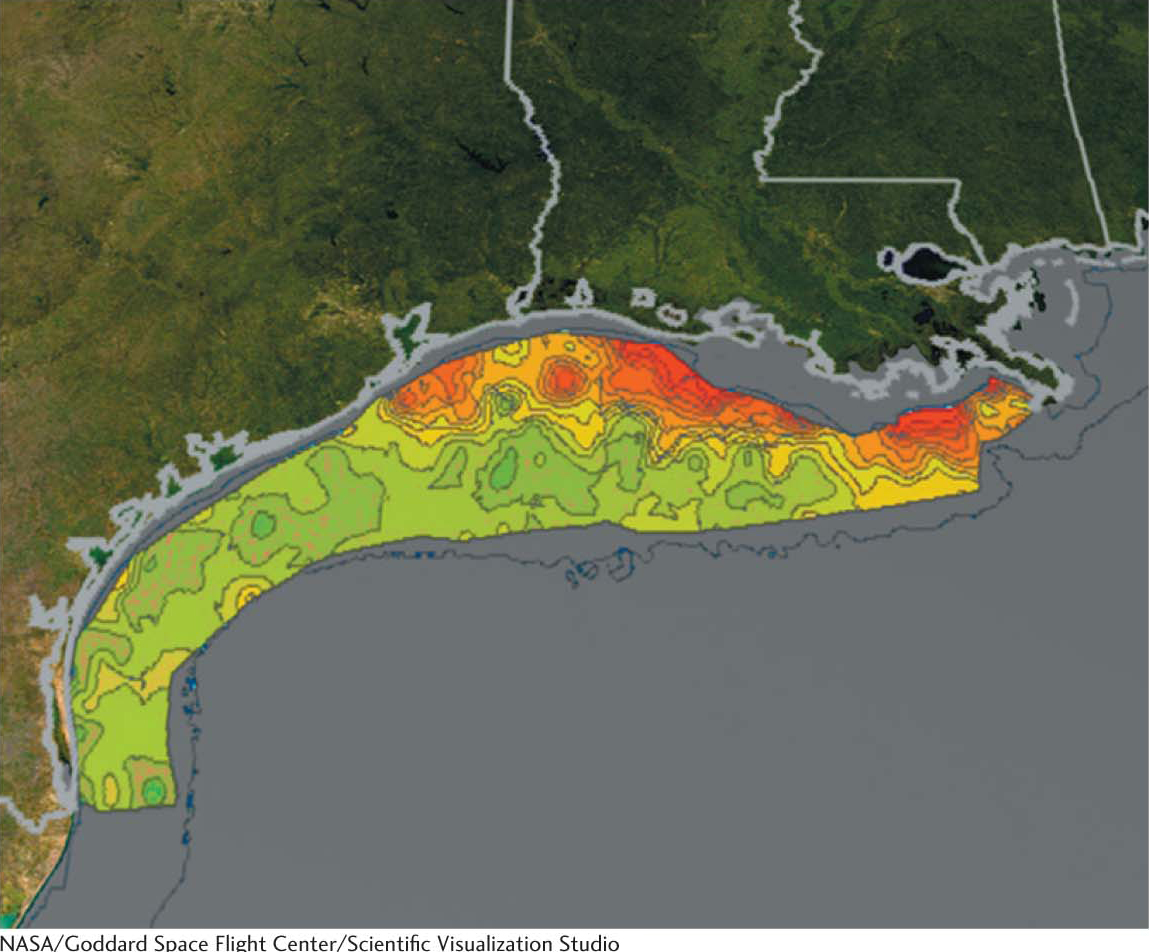
BIOLOGICAL INSIGHTThe Dead Zone: Too Much Respiration
Some marine organisms perform so much cellular respiration, and therefore consume so much molecular oxygen, that the oxygen concentration in the water is decreased to a level that is too low to sustain other organisms. One such hypoxic (low levels of oxygen) zone is in the northern Gulf of Mexico, off the coast of Louisiana where the Mississippi River flows into the Gulf (Figure 20.16). The Mississippi is extremely nutrient rich due to agricultural runoff; so plant microorganisms, called phytoplankton, proliferate so robustly that they exceed the amount that can be consumed by other members of the food chain. When the phytoplankton die, they sink to the bottom and are consumed by aerobic bacteria. The aerobic bacteria thrive to such a degree that other bottom-
Toxic Derivatives of Molecular Oxygen Such As Superoxide Radical Are Scavenged by Protective Enzymes
Molecular oxygen is an ideal terminal electron acceptor because its high affinity for electrons provides a large thermodynamic driving force. However, the reduction of O2 can result in dangerous side reactions. The transfer of four electrons leads to safe products (two molecules of H2O), but partial reduction generates hazardous compounds. In particular, the transfer of a single electron to O2 forms superoxide ion, whereas the transfer of two electrons yields peroxide:
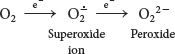
From 2% to 4% of the oxygen molecules consumed by mitochondria are converted into superoxide ion, predominantly at Complexes I and III. Superoxide, peroxide, and species that can be generated from them such as the hydroxyl radical (OH∙) are collectively referred to as reactive oxygen species or ROS. Reactive oxygen species react with essentially all macromolecules in the cell, including proteins, nucleotide bases, and membranes. Oxidative damage caused by ROS has been implicated in the aging process as well as in a growing list of diseases (Table 20.3).
What are the cellular defense strategies against oxidative damage by ROS? Chief among them is the enzyme superoxide dismutase. This enzyme scavenges superoxide radicals by catalyzing the conversion of two of these radicals into hydrogen peroxide and molecular oxygen:
DID YOU KNOW?
In mammals, the mutation rate for mitochondrial DNA is 10-

DID YOU KNOW?
Dismutation is a reaction in which a single reactant is converted into two different products.
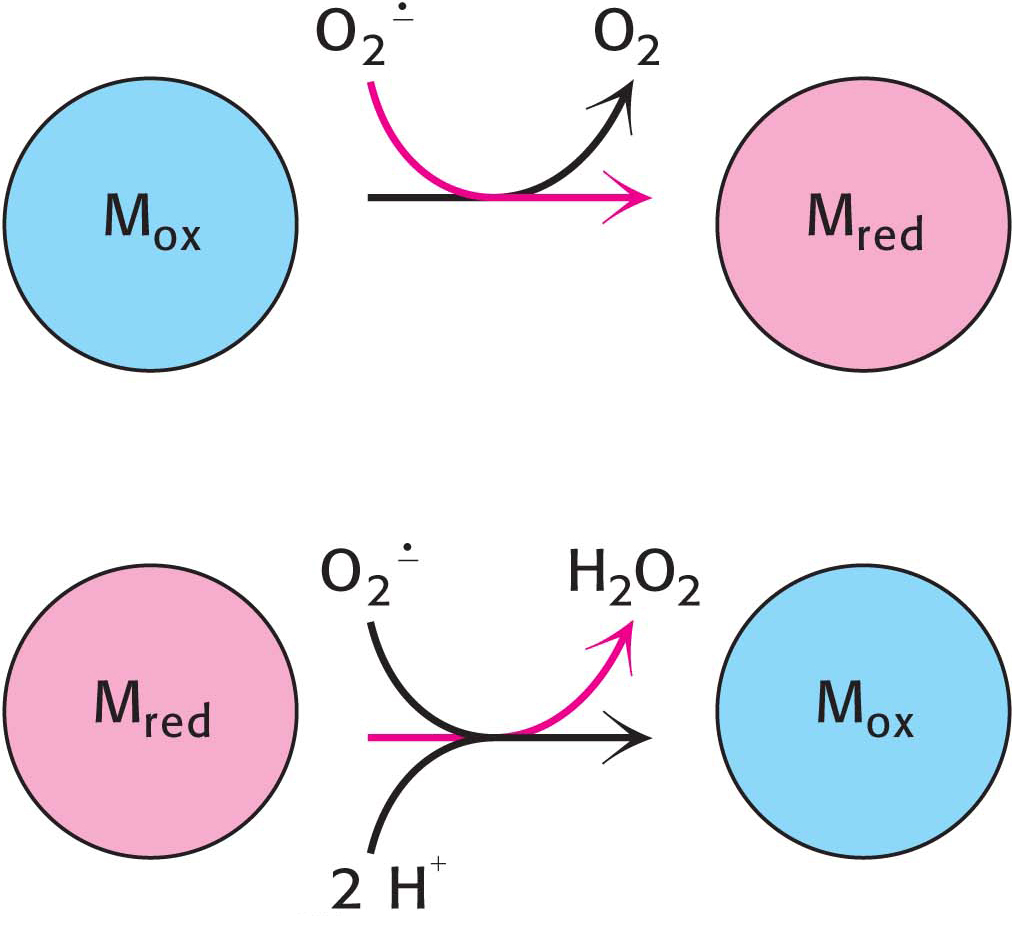
This reaction takes place in two steps. The oxidized form of the enzyme is reduced by superoxide to form oxygen (Figure 20.17). The reduced form of the enzyme then reacts with a second superoxide ion to form peroxide, which takes up two protons along the reaction path to yield hydrogen peroxide.
The hydrogen peroxide formed by superoxide dismutase and by other processes is scavenged by catalase, a ubiquitous heme protein that catalyzes the dismutation of hydrogen peroxide into water and molecular oxygen:

Superoxide dismutase and catalase are remarkably efficient, performing their reactions at or near the diffusion-
A long-
Despite the fact that reactive oxygen species are known hazards, recent evidence suggests that the controlled generation of these molecules may be important components of signal-