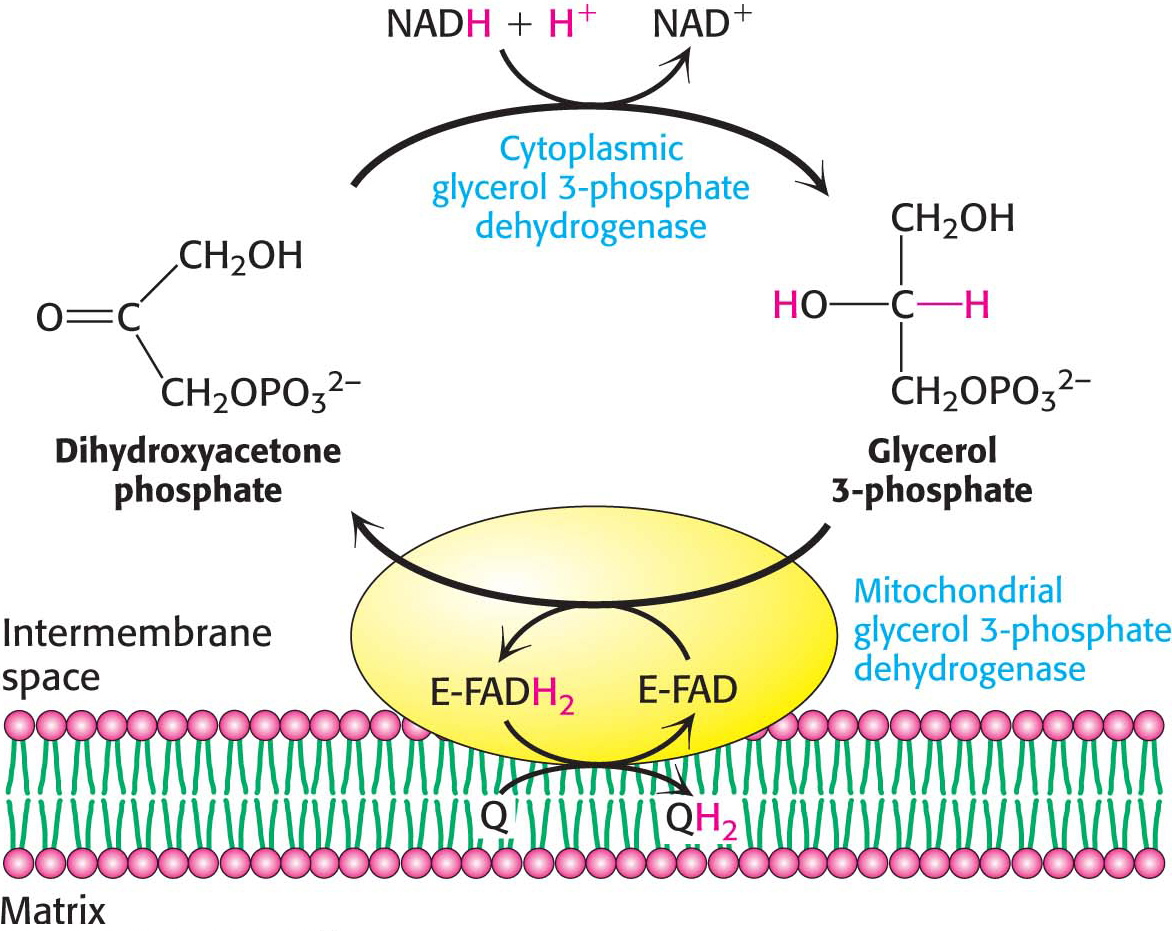
Electrons from Cytoplasmic NADH Enter Mitochondria by Shuttles
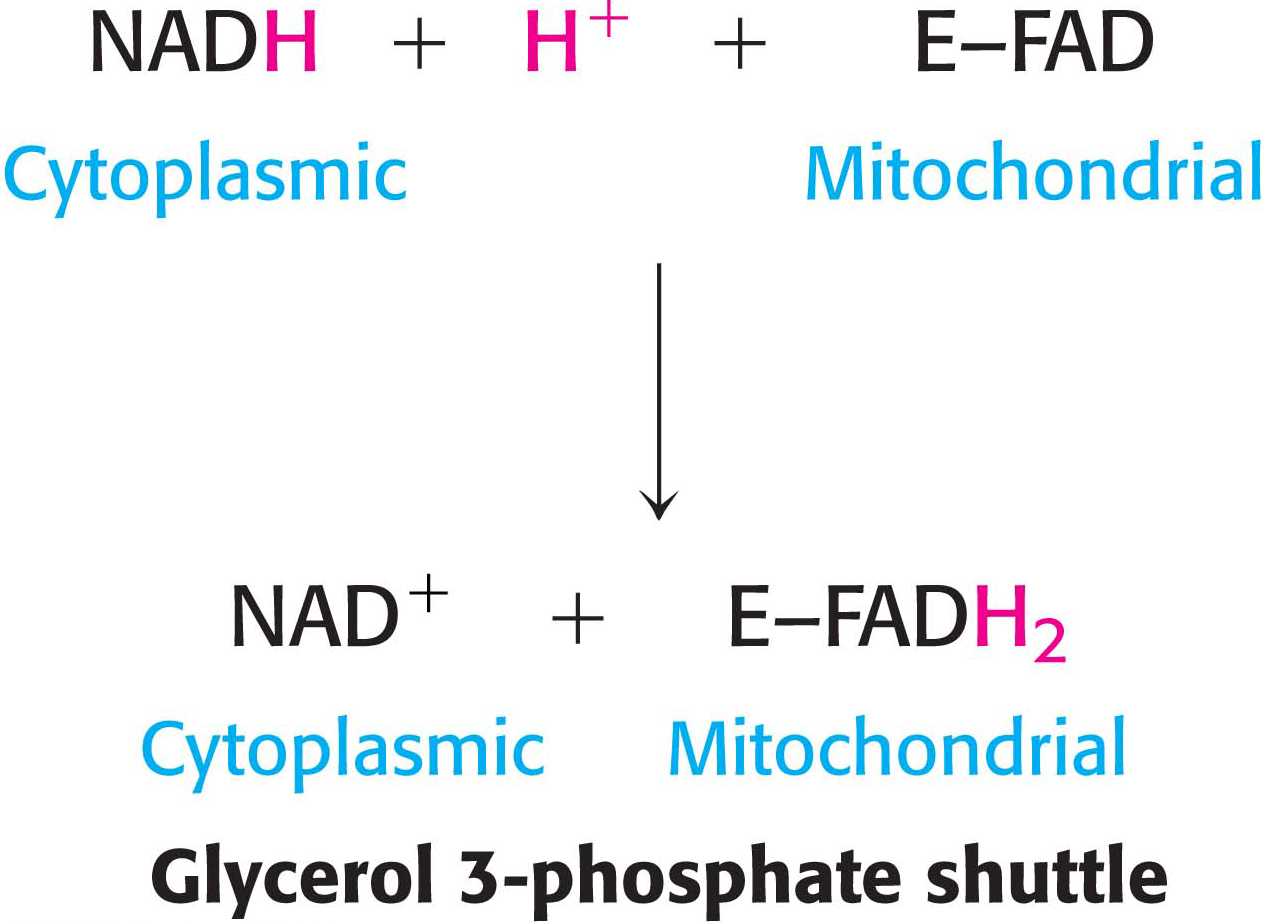
One function of the respiratory chain is to regenerate NAD+ for use in glycolysis. NADH generated by the citric acid cycle and fatty acid oxidation is already in the mitochondrial matrix, but how is cytoplasmic NADH reoxidized to NAD+ under aerobic conditions? NADH cannot simply pass into mitochondria for oxidation by the respiratory chain, because the inner mitochondrial membrane is impermeable to NADH and NAD+. The solution is that electrons from NADH, rather than NADH itself, are carried across the mitochondrial membrane. One of several means of introducing electrons from NADH into the electron-transport chain is the glycerol 3-phosphate shuttle (Figure 21.12). The first step in this shuttle is the transfer of a pair of electrons from NADH to dihydroxyacetone phosphate, a glycolytic intermediate, to form glycerol 3-phosphate. This reaction is catalyzed by a glycerol 3-phosphate dehydrogenase in the cytoplasm. Glycerol 3-phosphate is reoxidized to dihydroxyacetone phosphate on the outer surface of the inner mitochondrial membrane by a membrane-bound isozyme of glycerol 3-phosphate dehydrogenase. An electron pair from glycerol 3-phosphate is transferred to an FAD prosthetic group in this enzyme to form FADH2. This reaction also regenerates dihydroxyacetone phosphate.
Page 391
The reduced flavin transfers its electrons to the electron carrier Q, which then enters the respiratory chain as QH2. When cytoplasmic NADH transported by the glycerol 3-phosphate shuttle is oxidized by the respiratory chain, 1.5 rather than 2.5 molecules of ATP are formed. The yield is lower because the electrons from cytoplasmic NADH are carried to the electron-transport chain by FAD. The use of FAD enables electrons from cytoplasmic NADH to be transported into mitochondria against an NADH concentration gradient that is formed because the reduced cofactors build up in the mitochondria when oxygen demand is high. The price of this transport is one molecule of ATP per two electrons. This glycerol 3-phosphate shuttle is especially prominent in muscle and enables it to sustain a very high rate of oxidative phosphorylation. Indeed, some insects lack lactate dehydrogenase and are completely dependent on the glycerol 3-phosphate shuttle for the regeneration of cytoplasmic NAD+.
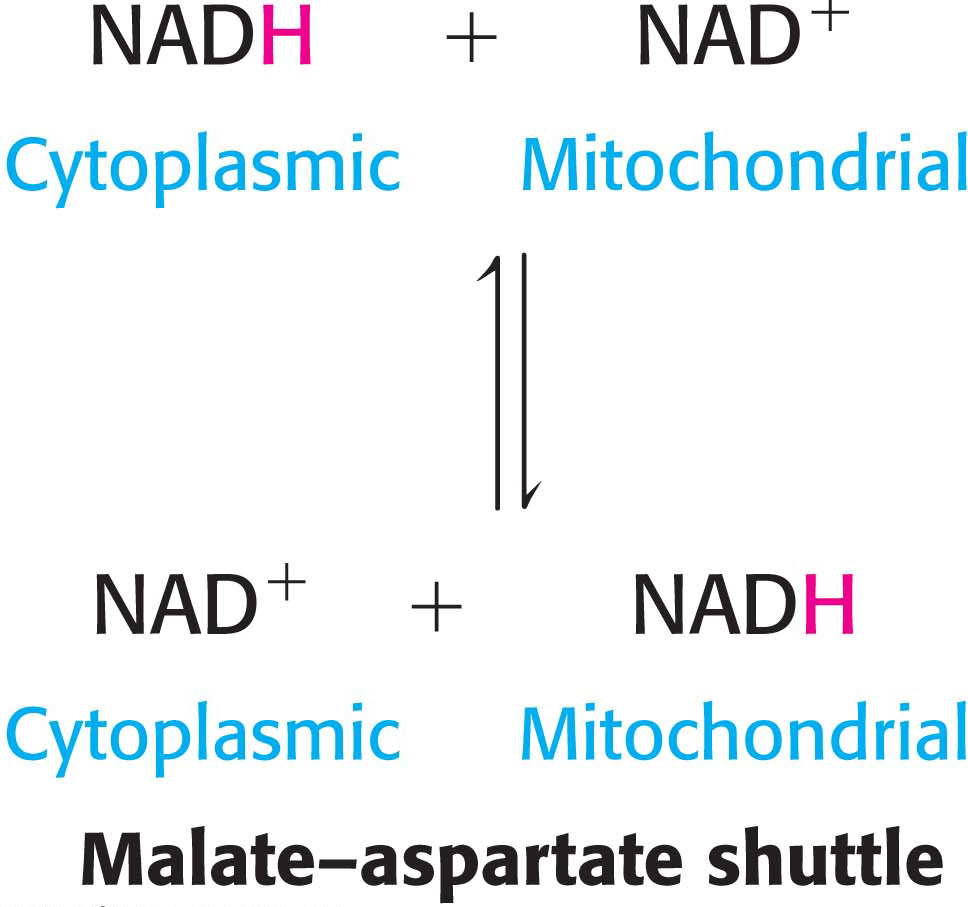
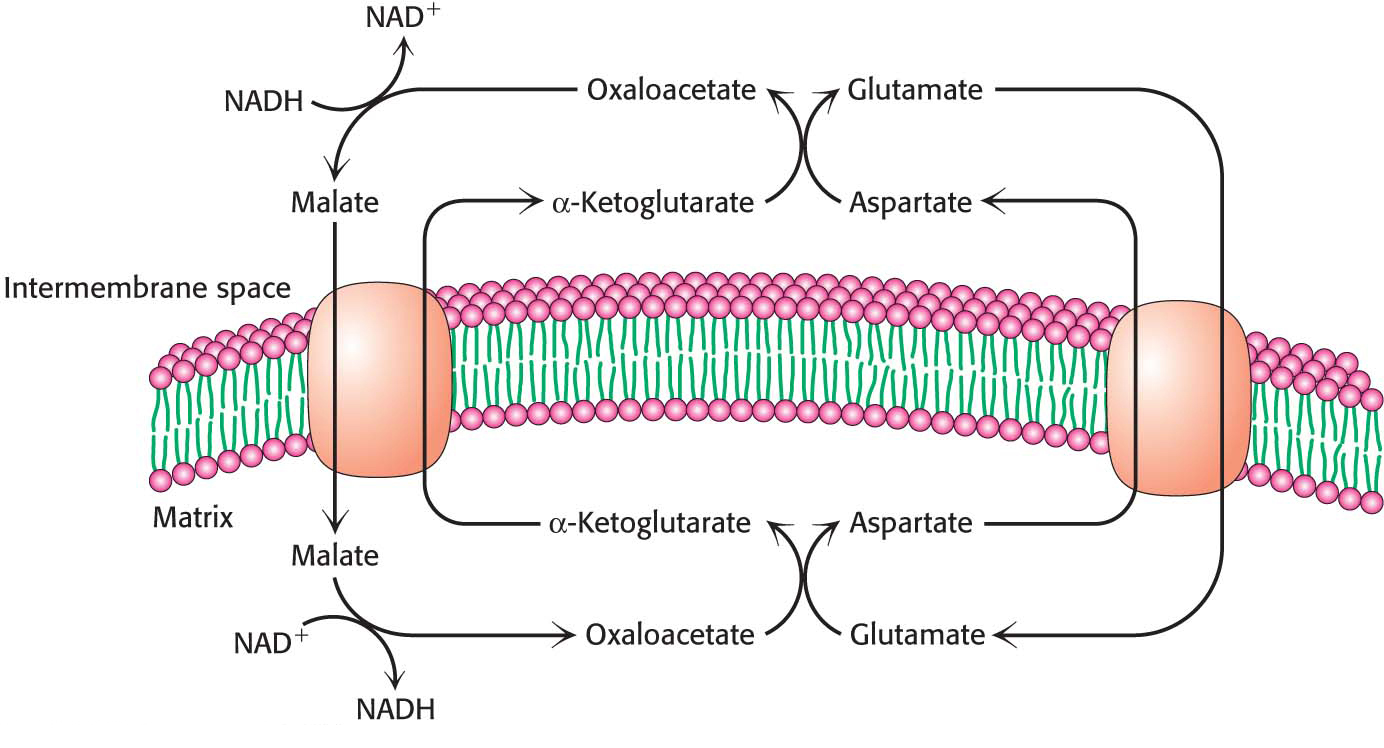
In the heart and liver, electrons from cytoplasmic NADH are brought into mitochondria by the malate–aspartate shuttle, which is mediated by two membrane carriers and four enzymes (Figure 21.13). Electrons are transferred from NADH in the cytoplasm to oxaloacetate, forming malate, which traverses the inner mitochondrial membrane in exchange for α-ketoglutarate by an antiporter. The malate is then reoxidized to oxaloacetate by NAD+ in the matrix to form NADH in a reaction catalyzed by the citric acid cycle enzyme malate dehydrogenase. The resulting oxaloacetate does not readily cross the inner mitochondrial membrane, and so a transamination reaction (Chapter 30) is needed to form aspartate, which can be transported to the cytoplasmic side by another antiporter in exchange for glutamate. Glutamate donates an amino group to oxaloacetate, forming aspartate and α-ketoglutarate. In the cytoplasm, aspartate is then deaminated to form oxaloacetate and the cycle is restarted.
Page 392
The Entry of ADP into Mitochondria Is Coupled to the Exit of ATP
The major function of oxidative phosphorylation is to generate ATP from ADP. However, these nucleotides do not diffuse freely across the inner mitochondrial membrane. How are these highly charged molecules moved across the inner membrane into the cytoplasm? A specific transport protein, ATP-ADP translocase (also called the adenine nucleotide transporter, ANT) enables these molecules to transverse this permeability barrier. Most importantly, the flows of ATP and ADP are coupled. ADP enters the mitochondrial matrix only if ATP exits, and vice versa. This process is carried out by the translocase, an antiporter:

ATP-ADP translocase is highly abundant, constituting about 15% of the protein in the inner mitochondrial membrane. The abundance is a manifestation of the fact that human beings exchange the equivalent of their weight in ATP each day. Despite the fact that the mitochondria are the sites of ATP synthesis, both the cytoplasm and the nucleus have more ATP than the mitochondria, a testament to the efficiency of transport.
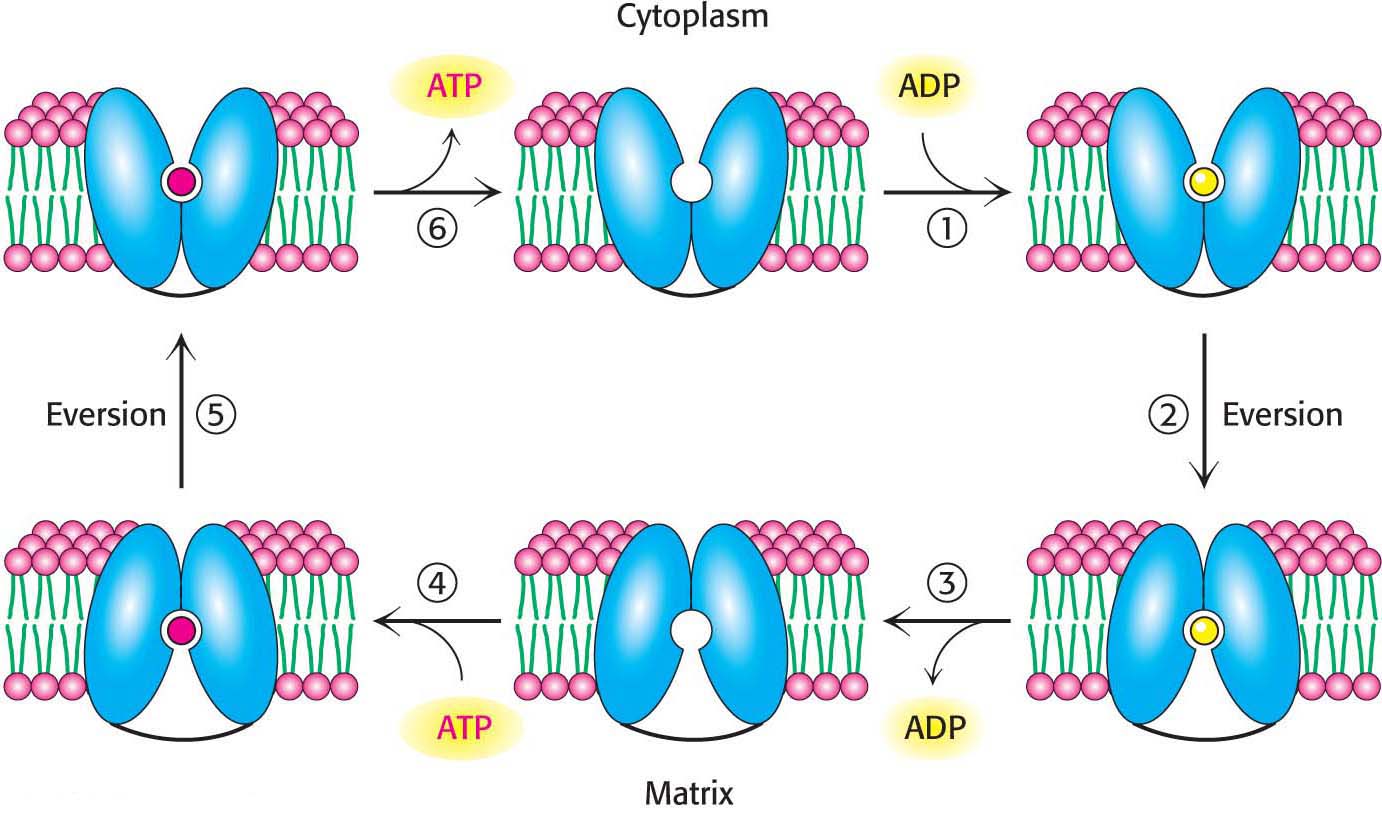
The 30-kDa translocase contains a single nucleotide-binding site that alternately faces the matrix and the cytoplasmic sides of the membrane (Figure 21.14). The key to transport is that ATP has one more negative charge than that of ADP. Thus, in an actively respiring mitochondrion with a positive membrane potential, ATP transport out of the mitochondrial matrix and ADP transport into the matrix are favored. This ATP–ADP exchange is not without significant energetic cost; about a quarter of the energy yield from electron transfer by the respiratory chain is consumed to regenerate the membrane potential that is tapped by this exchange process. The inhibition of ATP-ADP translocase leads to the subsequent inhibition of cellular respiration as well.
Page 393
Mitochondrial Transporters Allow Metabolite Exchange Between the Cytoplasm and Mitochondria
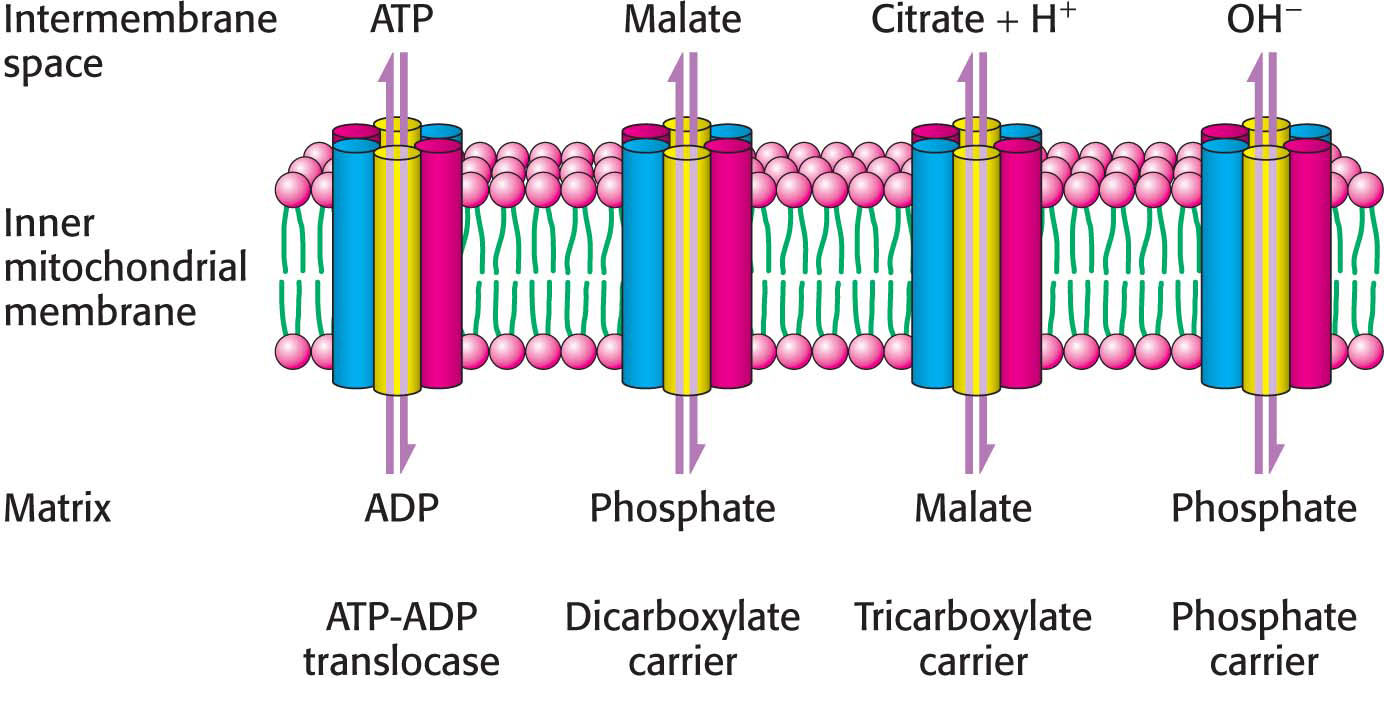
ATP-ADP translocase is only one of many mitochondrial transporters for ions and charged molecules (Figure 21.15). The phosphate carrier, which works in concert with ATP-ADP translocase, mediates the exchange of 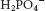 for OH−. The combined action of these two transporters leads to the exchange of cytoplasmic ADP and Pi for matrix ATP at the cost of the influx of one H+ (owing to the transport of one OH− out of the matrix, which lowers the proton gradient by binding an H+ to form water). These two transporters, which provide ATP synthase with its substrates, are associated with the synthase to form a large complex called the ATP synthasome.
for OH−. The combined action of these two transporters leads to the exchange of cytoplasmic ADP and Pi for matrix ATP at the cost of the influx of one H+ (owing to the transport of one OH− out of the matrix, which lowers the proton gradient by binding an H+ to form water). These two transporters, which provide ATP synthase with its substrates, are associated with the synthase to form a large complex called the ATP synthasome.
Other carriers also are present in the inner mitochondrial membrane. The dicarboxylate carrier enables malate, succinate, and fumarate to be exported from the mitochondrial matrix in exchange for Pi. The tricarboxylate carrier exchanges citrate and H+ for malate. Pyruvate in the cytoplasm enters the mitochondrial membrane in exchange for OH− by means of the pyruvate carrier. In all, more than 40 such carriers are encoded in the human genome.


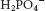 for OH−. The combined action of these two transporters leads to the exchange of cytoplasmic ADP and Pi for matrix ATP at the cost of the influx of one H+ (owing to the transport of one OH− out of the matrix, which lowers the proton gradient by binding an H+ to form water). These two transporters, which provide ATP synthase with its substrates, are associated with the synthase to form a large complex called the ATP synthasome.
for OH−. The combined action of these two transporters leads to the exchange of cytoplasmic ADP and Pi for matrix ATP at the cost of the influx of one H+ (owing to the transport of one OH− out of the matrix, which lowers the proton gradient by binding an H+ to form water). These two transporters, which provide ATP synthase with its substrates, are associated with the synthase to form a large complex called the ATP synthasome.