CHAPTER 22
The Light Reactions
Page 407
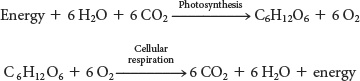
In Sections 8 and 9, we learned that ATP is generated through cellular respiration, the oxidation of the carbon atoms of carbohydrates, notably glucose, to CO2 with the reduction of O2 to water. In photosynthesis, this process must be reversed—reducing CO2 and oxidizing H2O to synthesize glucose:
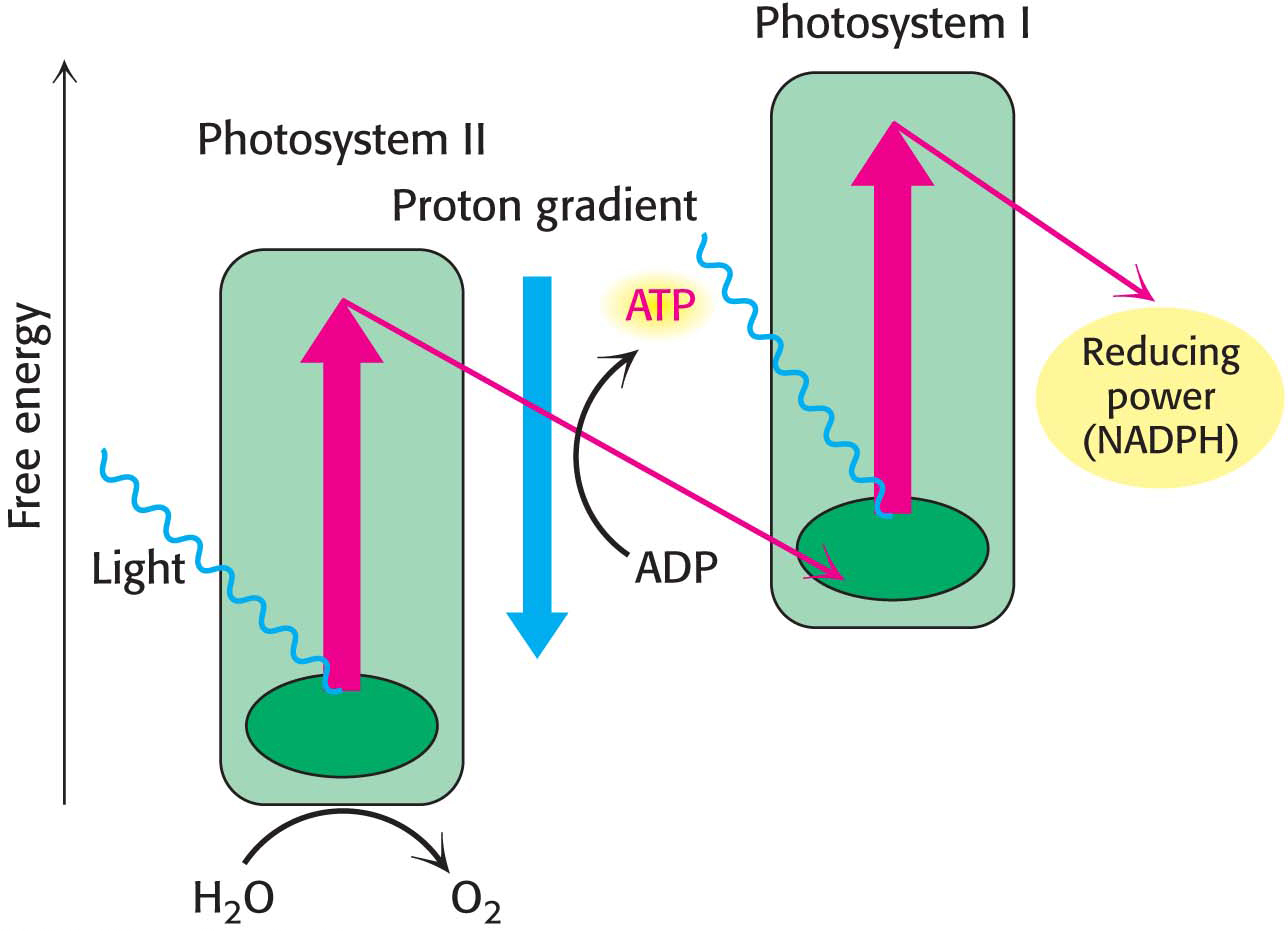
Although the processes of respiration and photosynthesis are chemically opposite each other, the biochemical principles governing the two processes are nearly identical. The key to both processes is the generation of high-energy electrons. The citric acid cycle oxidizes carbon fuels to CO2 to generate high-energy electrons. The flow of these high-energy electrons down an electron-transport chain generates a proton-motive force. This proton-motive force is then transduced by ATP synthase to form ATP. To synthesize glucose from CO2, high-energy electrons are required for two purposes: (1) to provide reducing power to reduce CO2 and (2) to generate ATP to power the reduction. How can high-energy electrons be generated without using a chemical fuel? Photosynthesis uses energy from light to boost electrons from a low-energy state to a high-energy state. In the high-energy, unstable state, nearby molecules can abscond with the excited electrons. These electrons are then used directly to produce biosynthetic reducing power in the form of NADPH, and they are used indirectly, through an electron-transport chain that generates a proton-motive force across a membrane, to drive the synthesis of ATP. The reactions that are powered by sunlight are called the light reactions (Figure 22.1).
Page 408
We begin with chloroplasts, the organelles in which photosynthesis takes place. We then examine the light-absorbing molecules that initiate the capture of light as high-energy electrons. Finally, we see how the high-energy electrons are used to form reducing power and ATP, the energy sources for the synthesis of glucose.