
22.2 Photosynthesis Transforms Light Energy into Chemical Energy
✓ 1 Describe the light reactions.
The role of chloroplasts in plants is to capture light energy. The trapping of light energy—
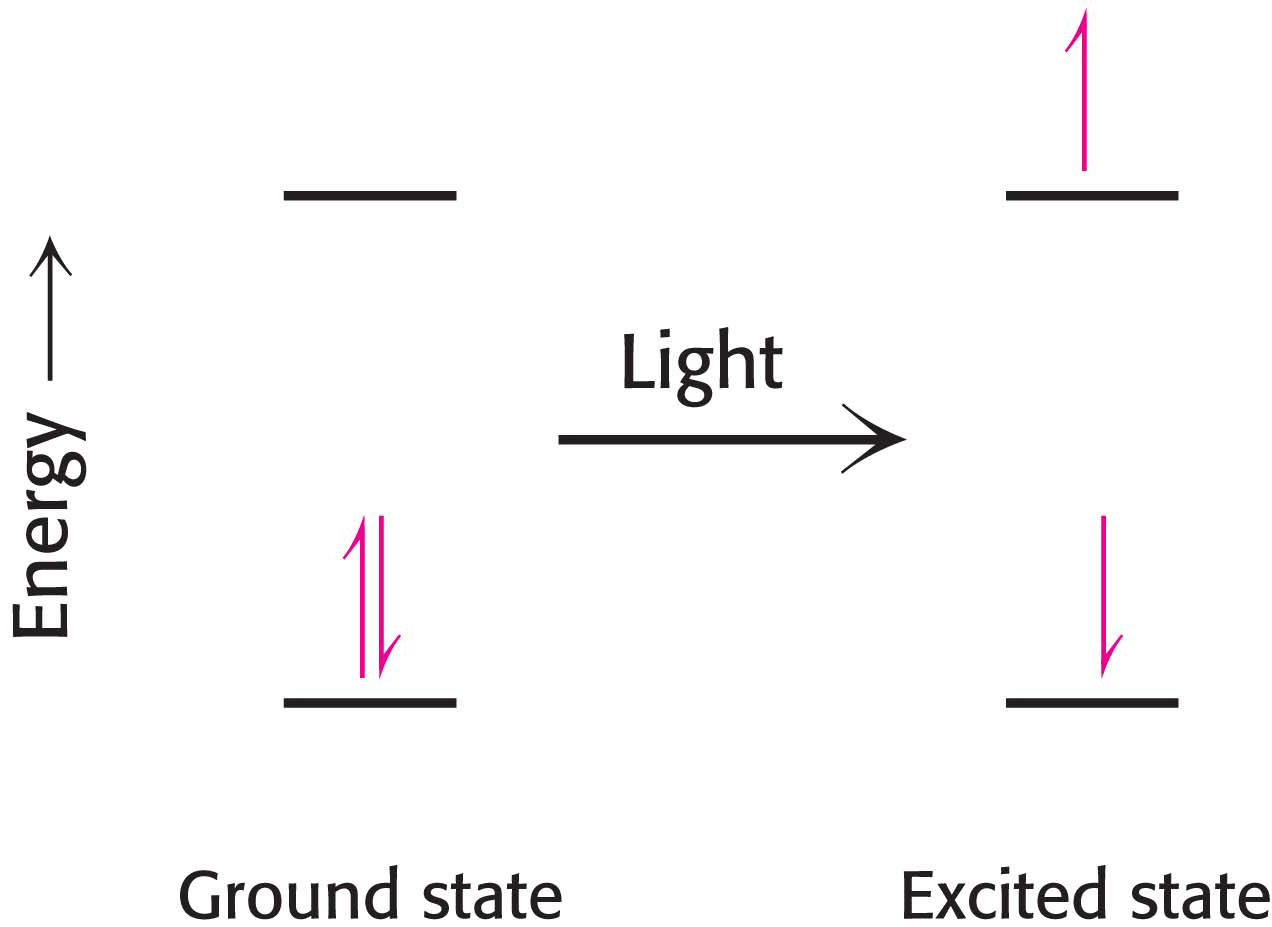
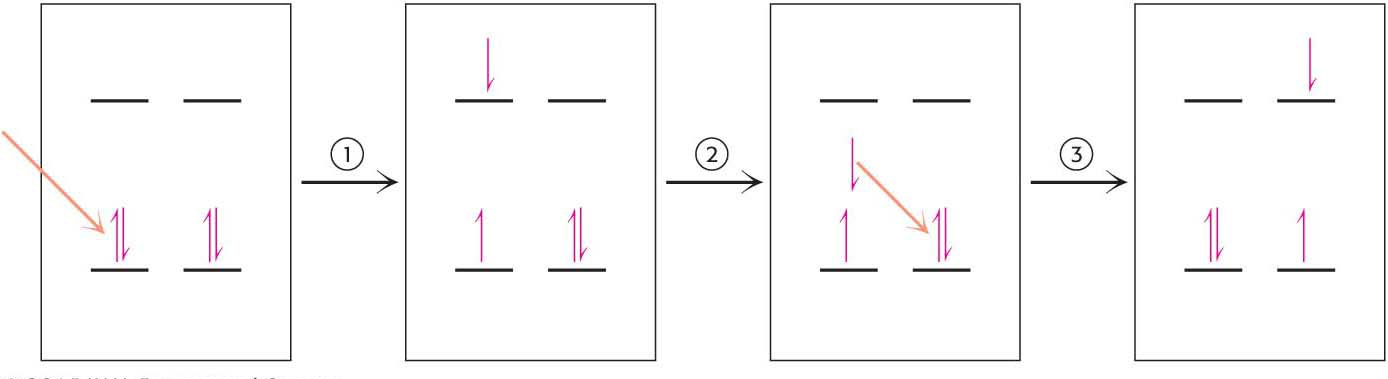
The first event in photosynthesis is the absorption of light by a photoreceptor molecule. Photoreceptor molecules, capable of absorbing the energy of light of a specific wavelength, are also called pigments. What happens when a photoreceptor molecule absorbs a photon of light of the appropriate energy? The energy from the light excites an electron from its ground energy level to an excited energy level (Figure 22.3). For most compounds that absorb light, the excited electron simply returns to the ground state and the absorbed energy is converted into heat or light, as when a molecule fluoresces. In photoreceptor molecules, however, the excitation energy released when one electron returns to its ground state may be accepted by an electron in a neighboring molecule, which then jumps to a higher energy state. This process of moving energy from one electron to another is called resonance energy transfer (Figure 22.4). Resonance energy transfer allows energy to be passed from one molecule to another, which, as we will see later, is an important property for increasing the efficiency of photosynthesis.
DID YOU KNOW?
A photon is a discrete bundle, or quantum, of electromagnetic energy that travels at the speed of light (c). The energy of a photon (E) is directly proportional to the frequency (v) of its vibration or inversely proportional to its wavelength (λ). The proportionality constant (h) is planck’s constant.
E = hv = hc/λ
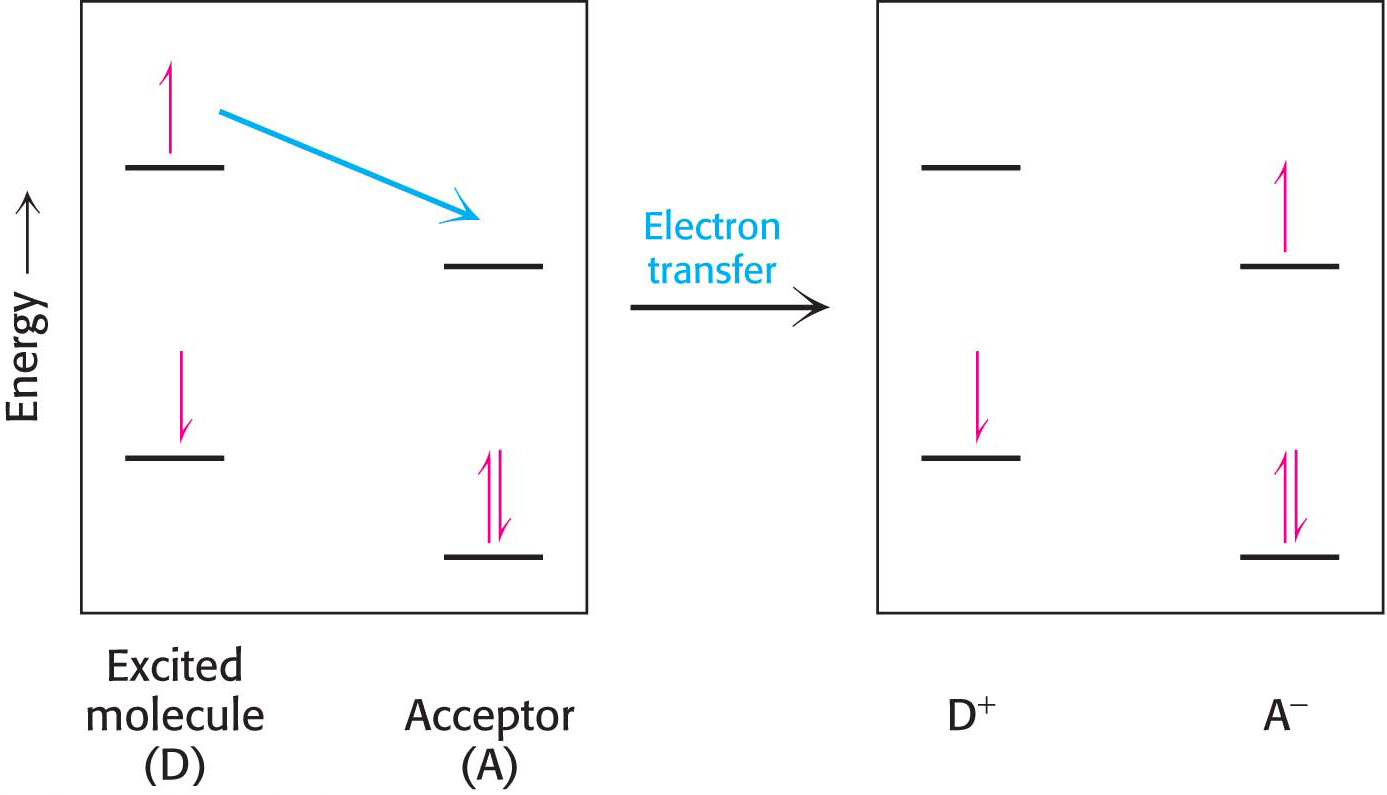
Alternatively, the excited electron itself may move to a nearby molecule that has a lower excited state, in a process called electron transfer (Figure 22.5). A positive charge forms on the initial molecule, owing to the loss of an electron, and a negative charge forms on the acceptor, owing to the gain of an electron. Hence, this process is referred to as photoinduced charge separation. The excited electron in its new molecule now has reducing power: it can reduce other molecules to store the energy originally obtained from light in chemical forms. The pair of electron carriers at which the charge separation takes place are called the special pair and are located at the reaction center.
Chlorophyll Is the Primary Light Acceptor in Most Photosynthetic Systems
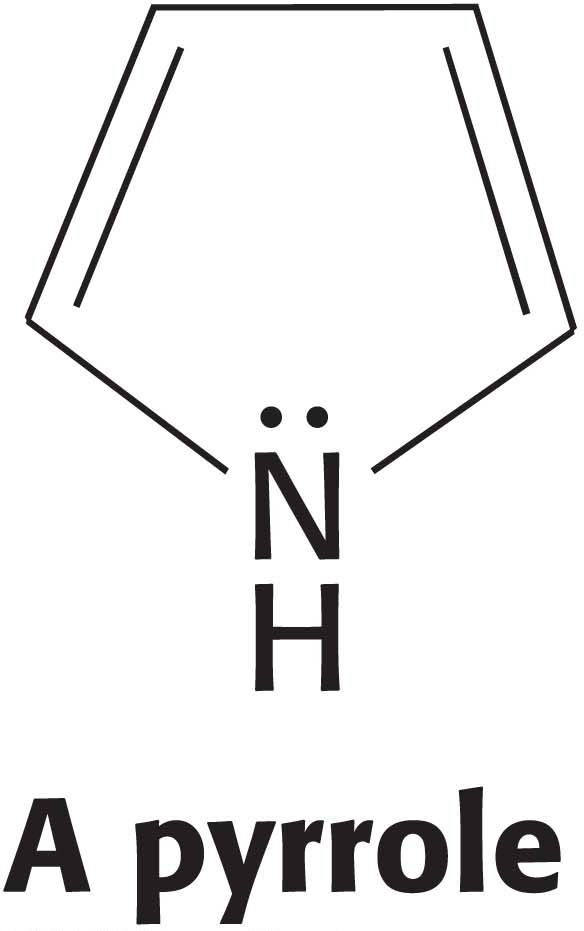
The principal photoreceptor in the chloroplasts of most green plants is chlorophyll, a substituted tetrapyrrole (Figure 22.6). The four nitrogen atoms of the pyrroles are bound to a magnesium ion, just as a heme group is bound to iron. A distinctive feature of chlorophyll is the presence of phytol, a highly hydrophobic 20-
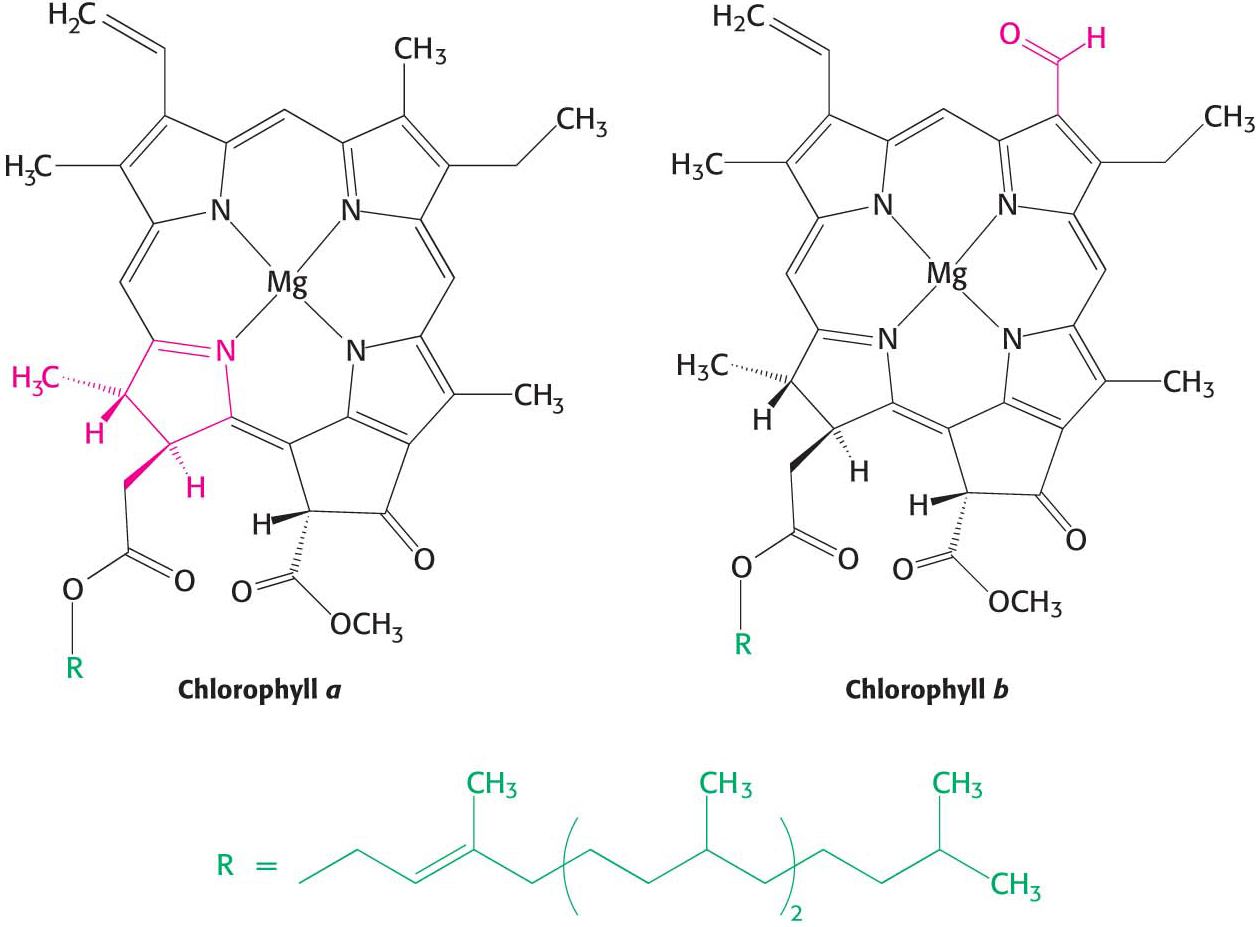
Chlorophylls are very effective photoreceptors because they contain networks of alternating single and double bonds. Such compounds are called polyenes and display resonance structures. Because the electrons are not held tightly to a particular atom and thus can resonate within the larger ring structure, they can be readily excited by light of suitable energy.
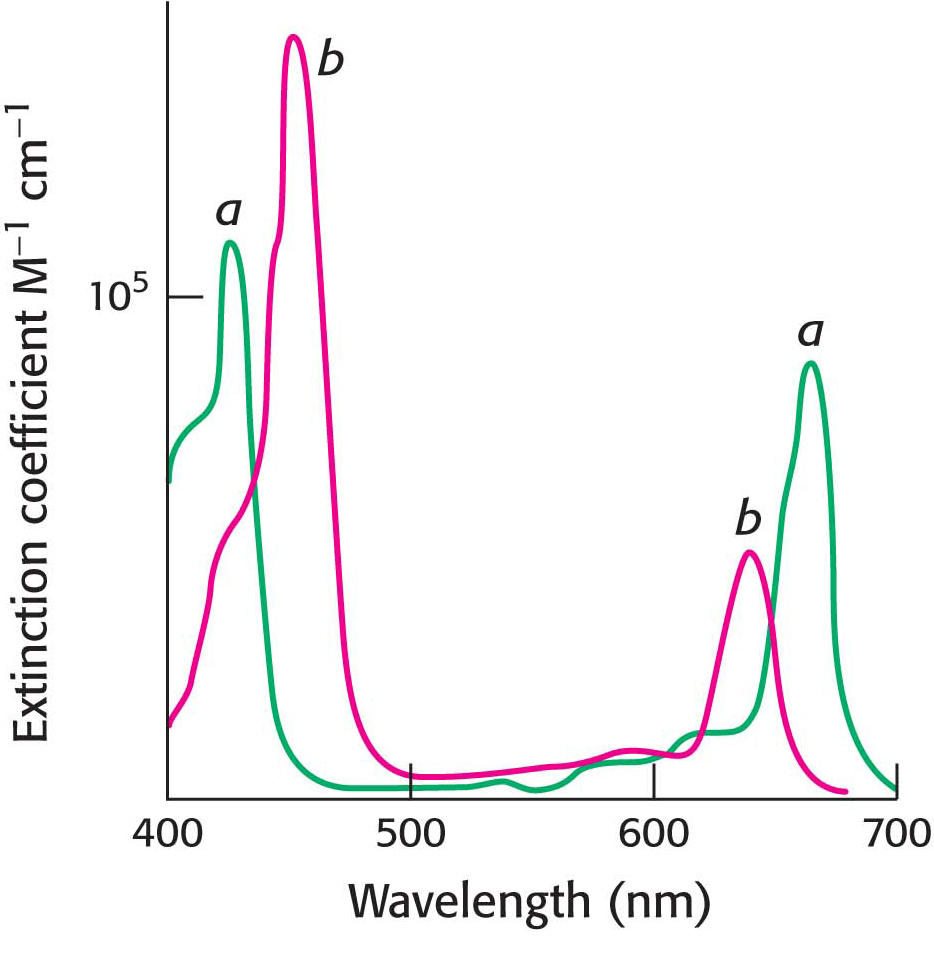
There are two primary varieties of chlorophyll molecules, chlorophyll a (Chla) and chlorophyll b (Chlb). Chlorophyll a, which is the principal chlorophyll in green plants, absorbs maximally at 420 nm and at 670 nm, leaving a large gap in the middle of the visible region. Chlorophyll b differs from chlorophyll a in having a formyl group in place of a methyl group. This small difference moves its two major absorption peaks toward the center of the visible region, where chlorophyll a does not absorb well (Figure 22.7).
The absorption of energy by chlorophyll in the reaction center, from electron transfer or directly from light, allows the chlorophyll molecule to donate an electron to a nearby molecule, its partner in the special pair, which results in photoinduced charge separation:
DID YOU KNOW?
“Life has set itself the task to catch the light on the wing to earth and to store the most elusive of all powers in rigid form.”
—Robert Julius Mayer, a German physician (1814–
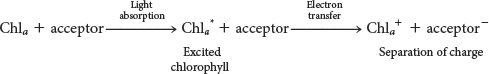
The negatively charged acceptor now has a high electron-
Light-Harvesting Complexes Enhance the Efficiency of Photosynthesis
A light-
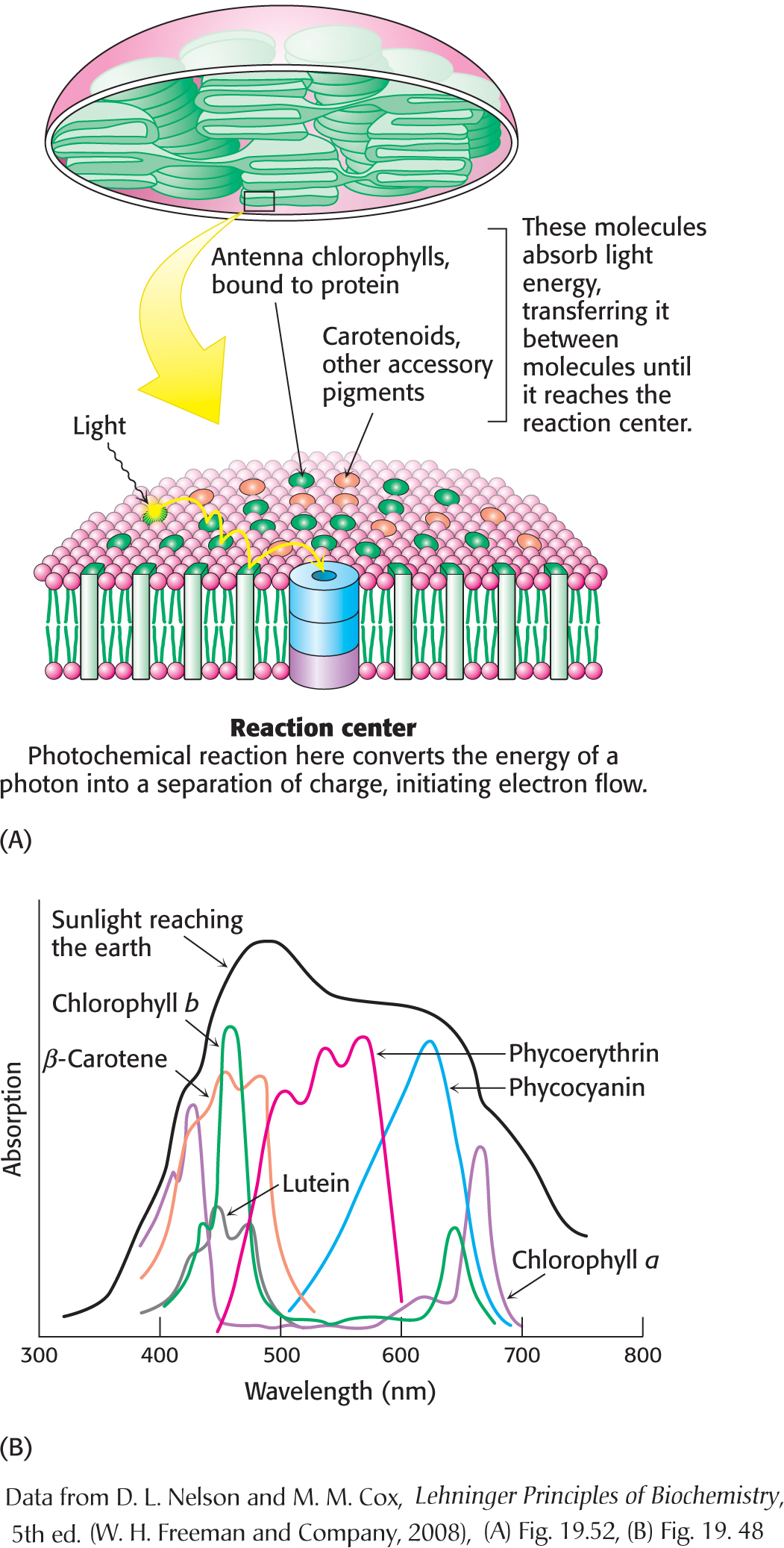
In addition to their role in transferring energy to reaction centers, the carotenoids and other accessory pigments serve a safeguarding function. Carotenoids suppress damaging photochemical reactions, particularly those including oxygen, that can be induced by bright sunlight. This protection may be especially important in the fall when the primary pigment chlorophyll is being degraded and thus not able to absorb light energy. Plants lacking carotenoids are quickly killed on exposure to light and oxygen.
 BIOLOGICAL INSIGHT
BIOLOGICAL INSIGHTChlorophyll in Potatoes Suggests the Presence of a Toxin
Chlorophyll synthesis is a warning sign when it comes to identifying poisonous potatoes. Light activates a noxious pathway in potatoes that leads to the synthesis of solanine, a toxic alkaloid. Plant alkaloids include such molecules as nicotine, caffeine, morphine, cocaine, and codeine.
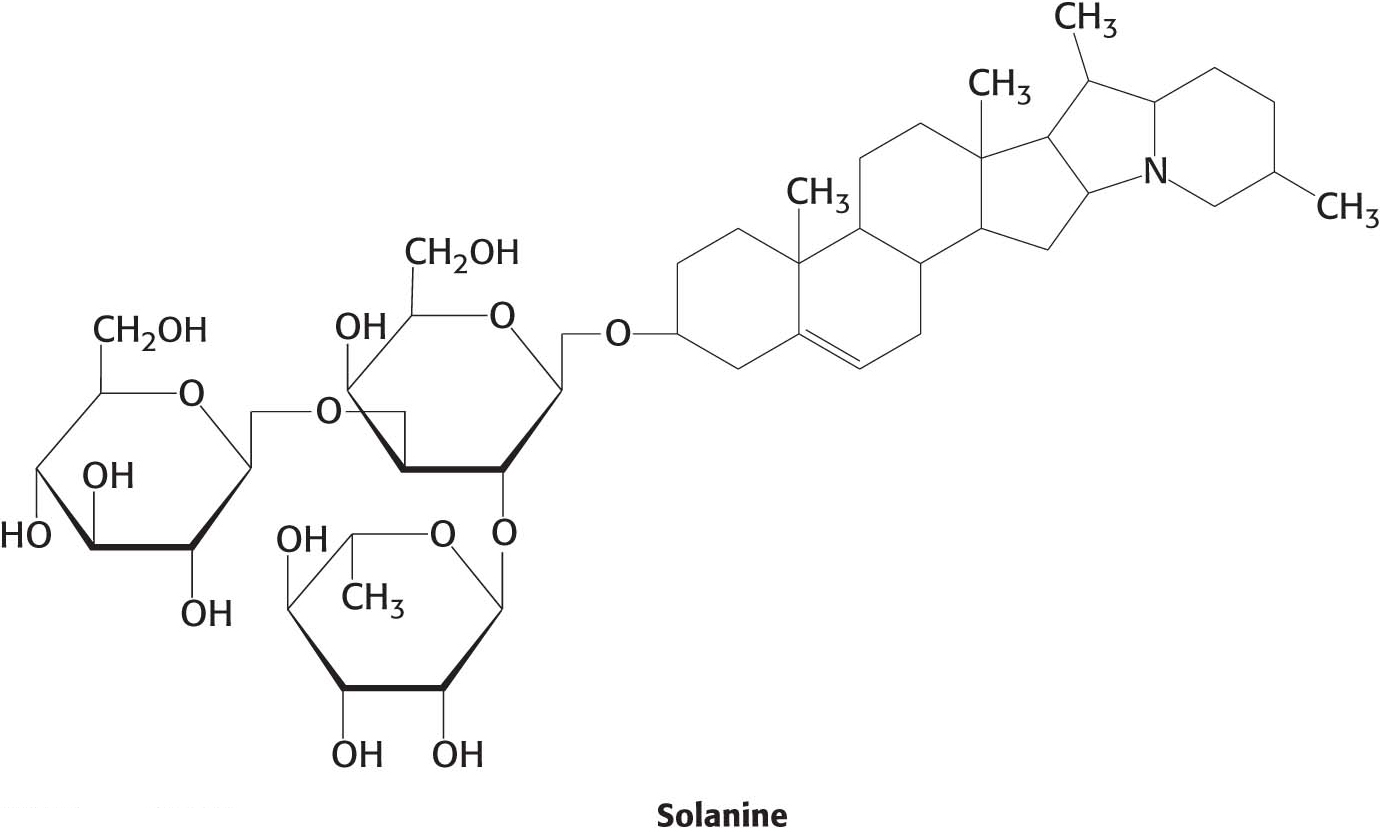
Solanine is toxic to animals because it inhibits acetylcholinesterase, an enzyme crucial for controlling the transmission of nerve impulses. Potato plants are thought to synthesize solanine to discourage insects from eating the potato. Light also causes potatoes to synthesize chlorophyll, which causes the tubers to turn green. Potatoes that are green have been exposed to light and are therefore probably also synthesizing solanine (Figure 22.9). For this reason, it is best not to eat green potatoes or potato chips with green edges.
