22.3 Two Photosystems Generate a Proton Gradient and NADPH
✓ 2 Identify the key products of the light reactions.
✓ 3 Explain how redox balance is maintained during the light reactions.
With an understanding of the principles of how photosynthetic organisms generate high-
Photosystem I Uses Light Energy to Generate Reduced Ferredoxin, a Powerful Reductant
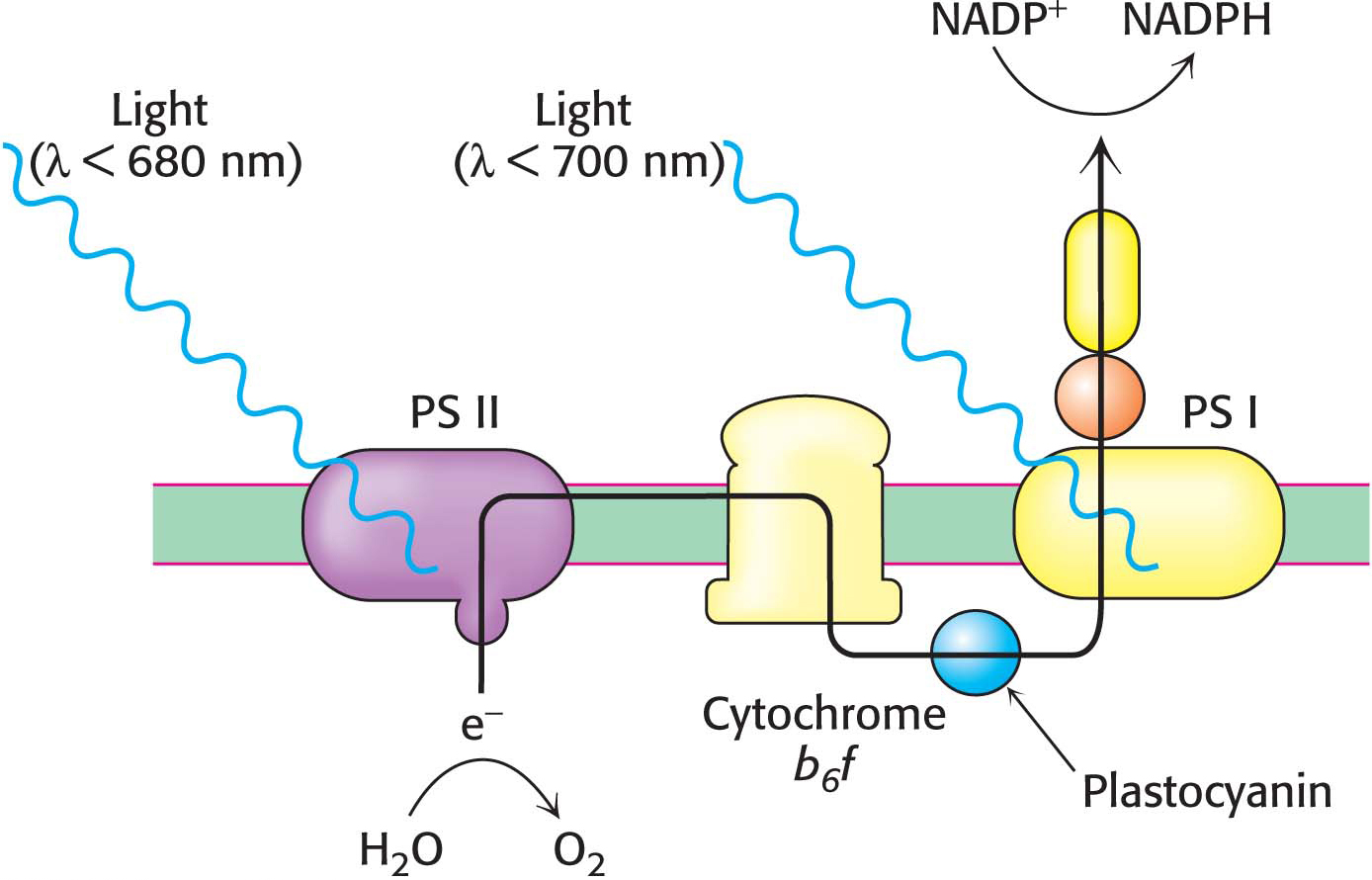
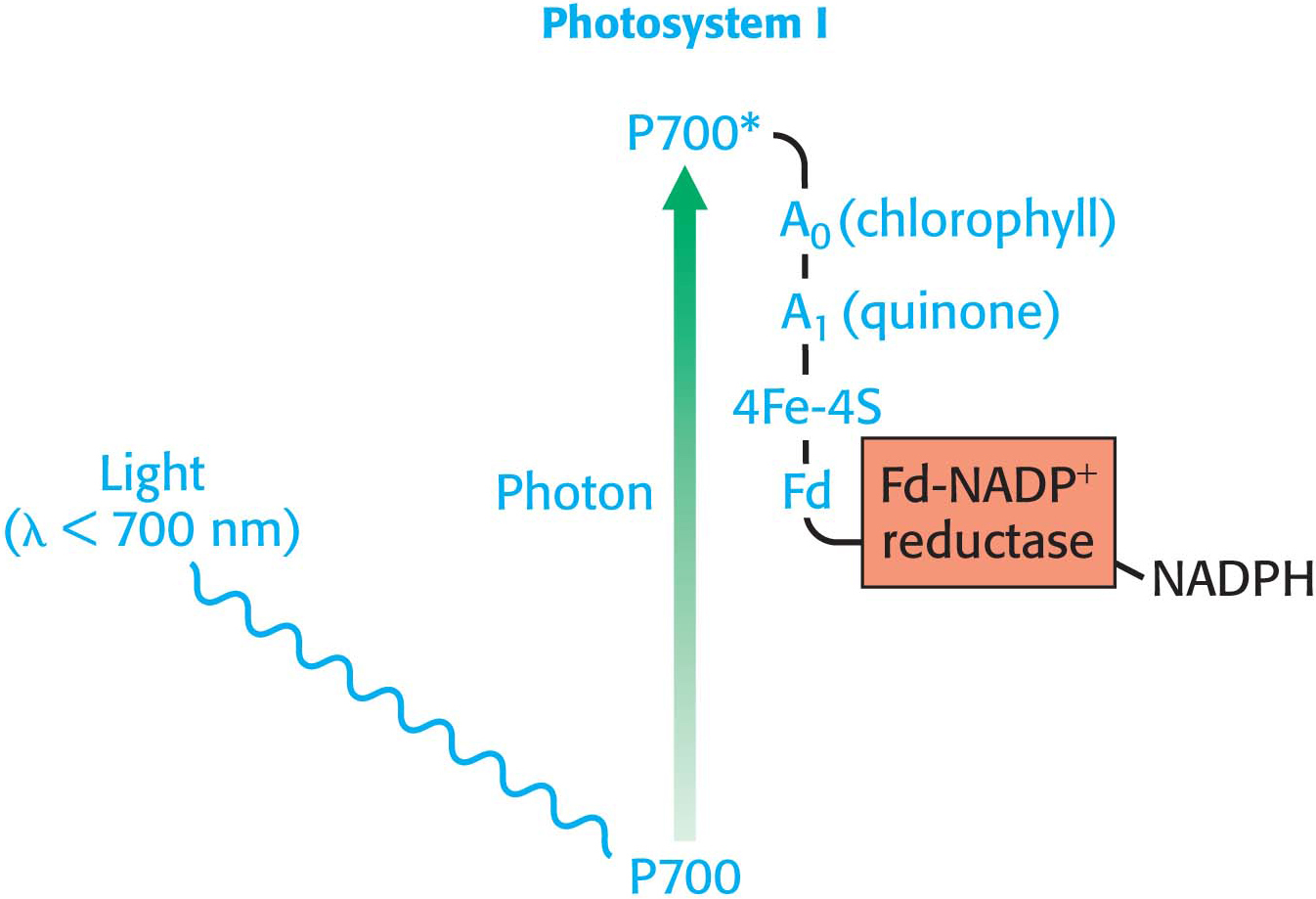
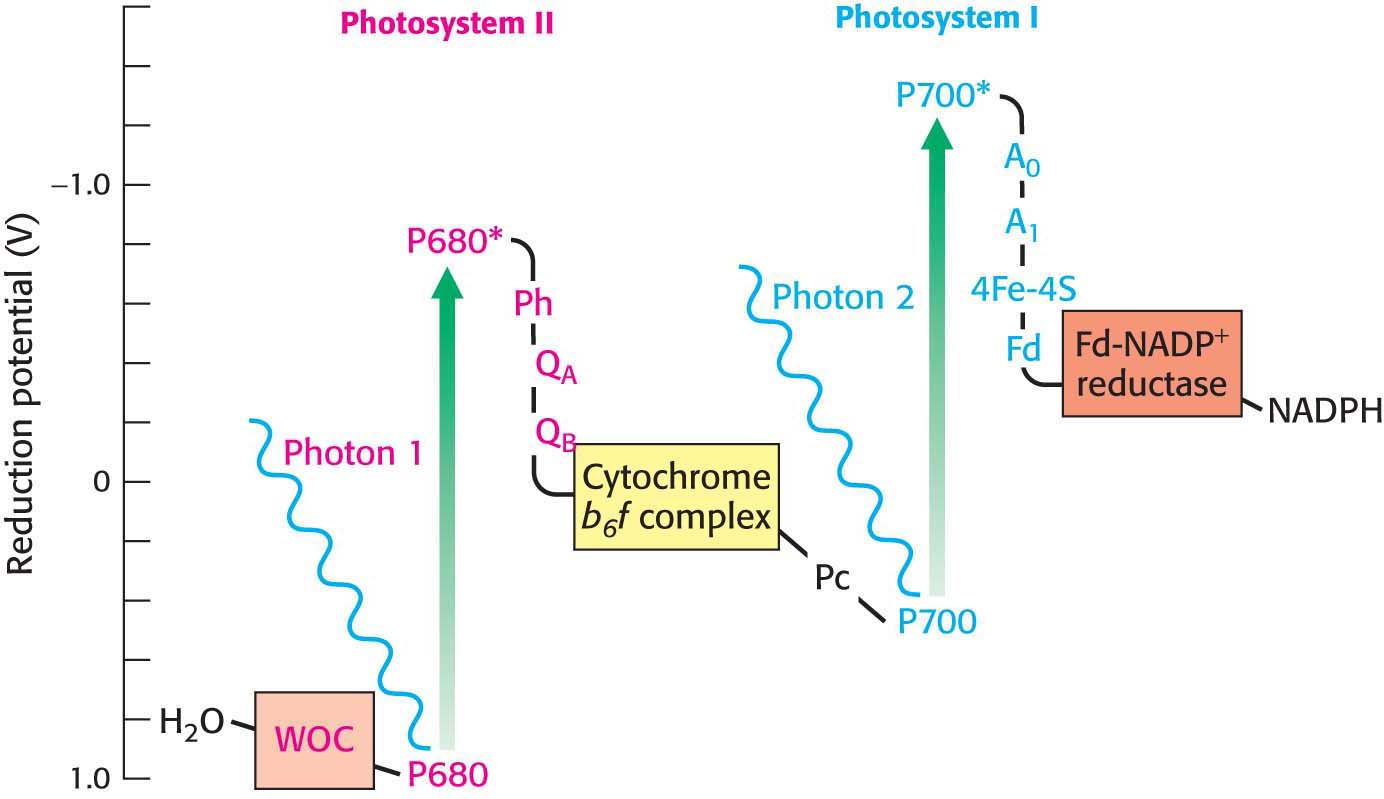
The first stage of the light reactions is catalyzed by photosystem I (Figure 22.11). Photosystem I typically includes 14 polypeptide chains and multiple associated proteins and cofactors. The core of this system is a pair of similar subunits, PsaA (83 kDa) and PsaB (82 kDa), which bind 80 chlorophyll molecules as well as other redox factors. A special pair of chlorophyll a molecules lies at the center of the structure and absorbs wavelengths of light that are longer than those absorbed by chlorophyll a not associated with a photosystem. The special pair of the reaction center of photosystem I are called P700. When activated by light (designated P700*), the reaction center initiates photoinduced charge separation that generates the high-
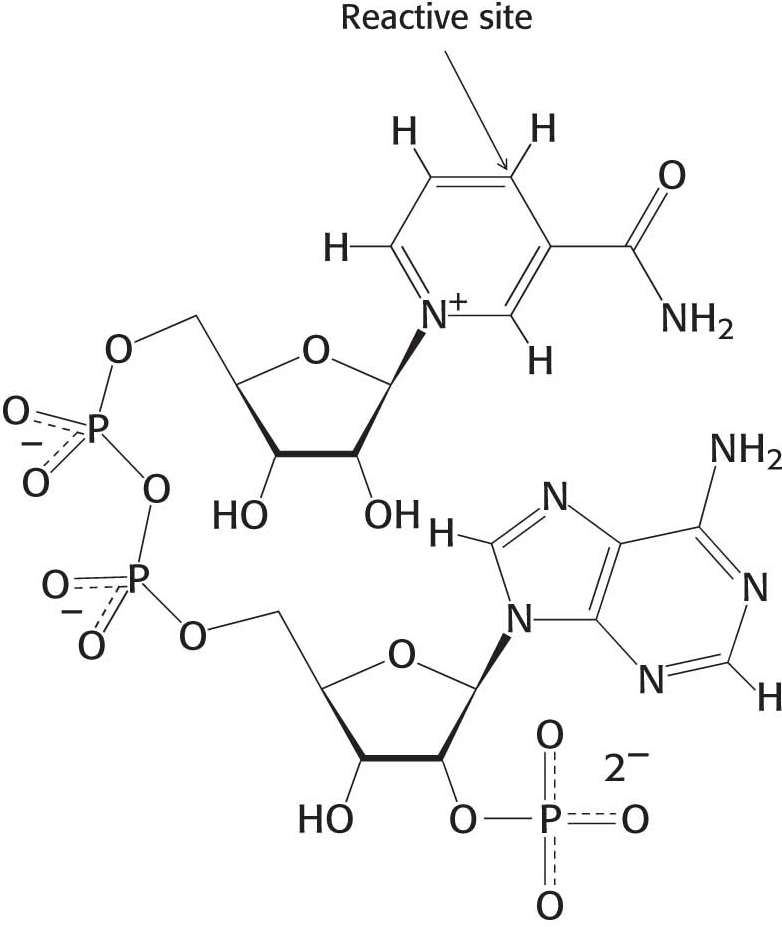
Although reduced ferredoxin is a strong reductant, it is not useful for driving many reactions, in part because ferredoxin carries only one available electron. The currency of readily available biosynthetic reducing power in cells is NADPH, which shuttles two electrons as a hydride ion. Indeed, NADPH functions exactly as NADH does and differs structurally only in that it contains a phosphoryl group on the 2′-hydroxyl group of one of the ribose units (Figure 22.13). This phosphoryl groups acts as a label identifying NADPH as a reductant for biosynthetic (anabolic) reactions, and so it is not oxidized by the respiratory chain as is NADH. This distinction holds true in all biochemical systems, including that of human beings.
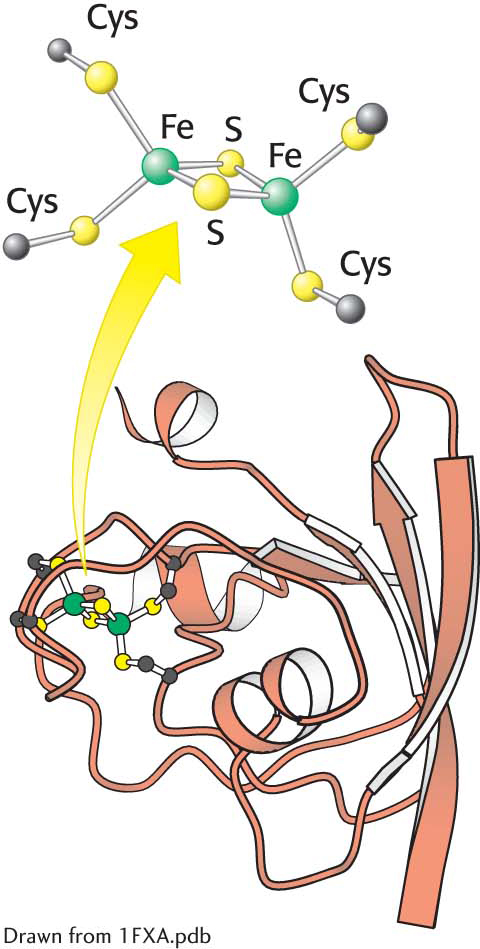
How can reduced ferredoxin be used to drive the reduction of NADP+ to NADPH? This reaction is catalyzed by ferredoxin-
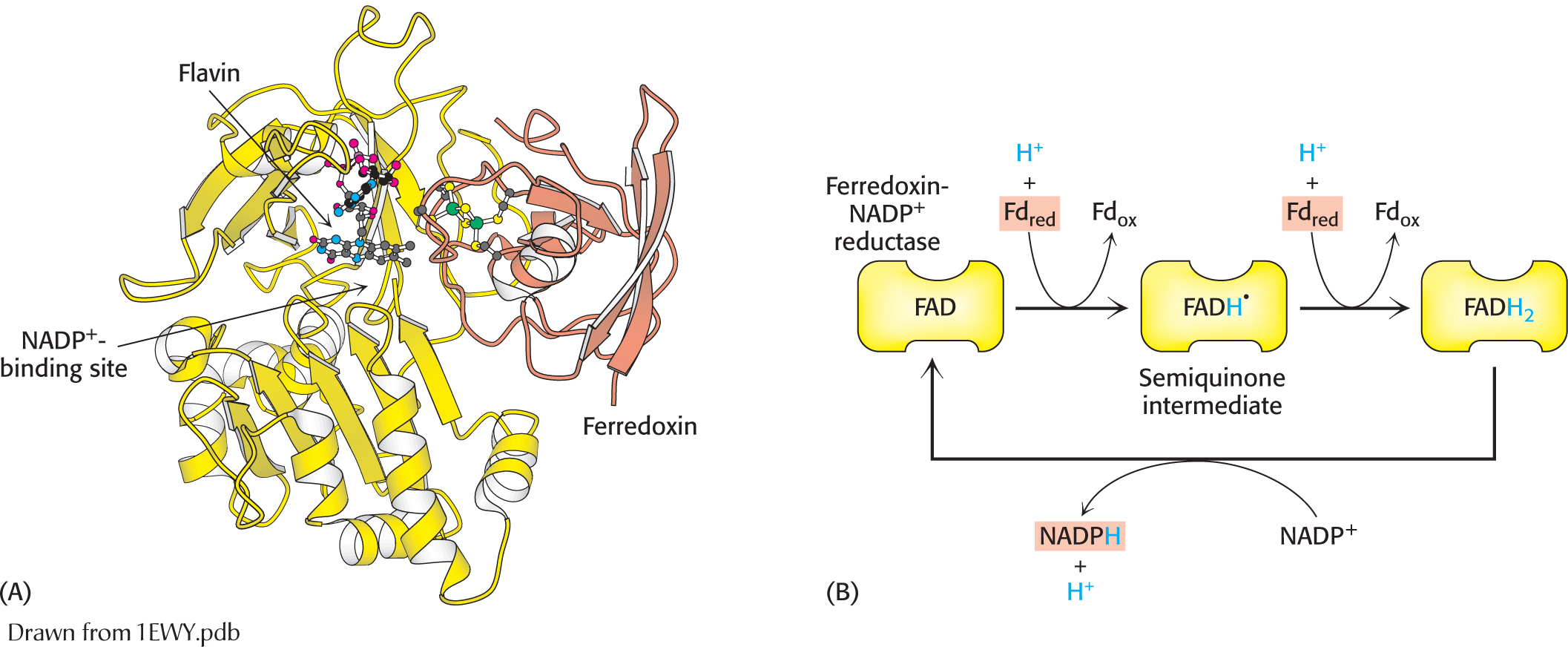
 The structure and function of ferredoxin-
The structure and function of ferredoxin-Photosystem II Transfers Electrons to Photosystem I and Generates a Proton Gradient
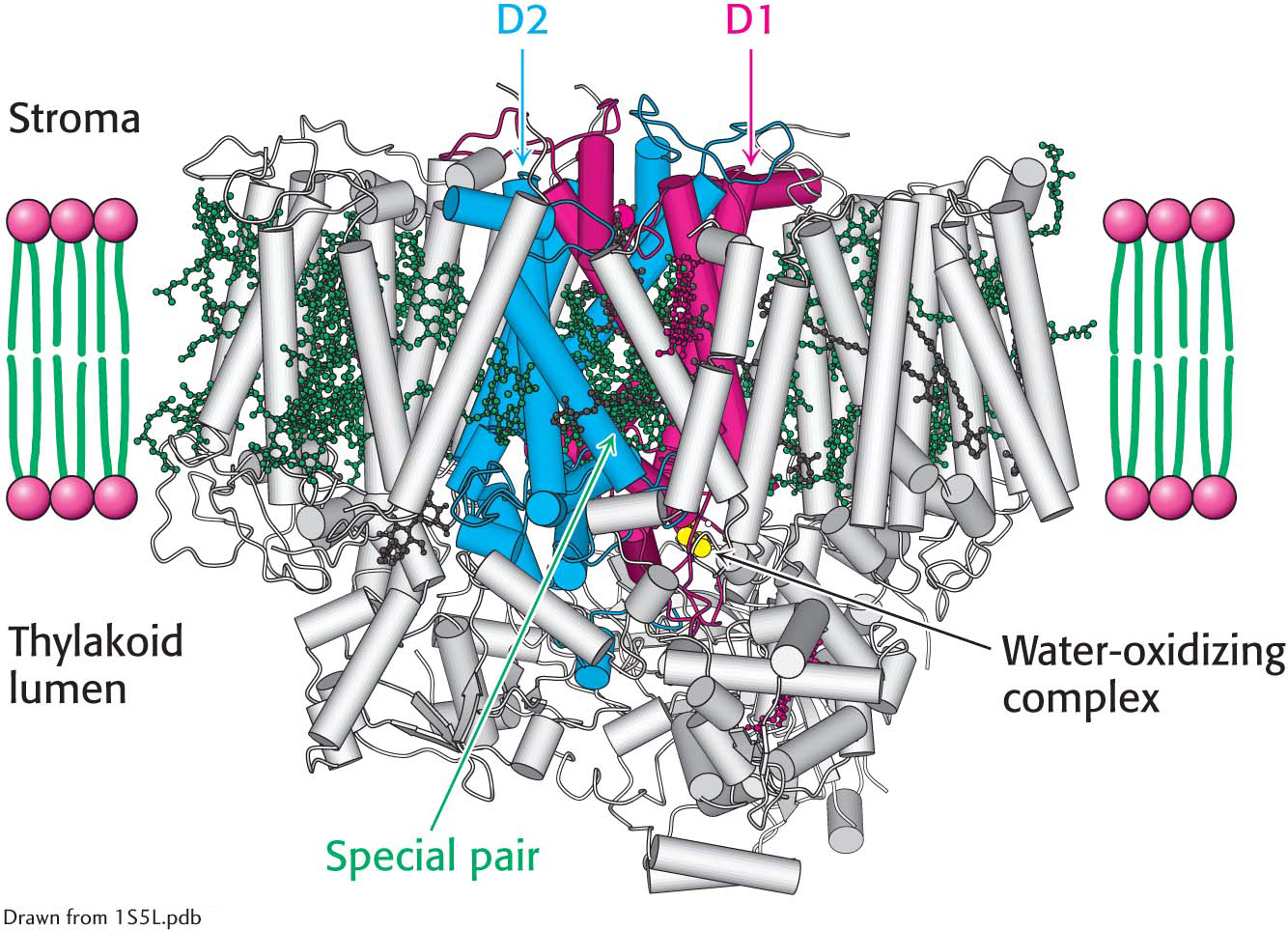
 The structure of photosystem II. The D1 and D2 subunits are shown in red and blue, respectively, and the numerous bound chlorophyll molecules are shown in dark green. Notice that the special pair and the water-
The structure of photosystem II. The D1 and D2 subunits are shown in red and blue, respectively, and the numerous bound chlorophyll molecules are shown in dark green. Notice that the special pair and the water-DID YOU KNOW?
Pheophytin is formed in the cooking of some green vegetables when the magnesium ion of chlorophyll is lost and replaced by protons. The absorption properties of pheophytin are different from those of the chlorophylls, often resulting in cooked vegetables that are a dull green compared with their uncooked counterparts.
Restocking the electrons of photosystem I requires another source of electrons. One of the roles of photosystem II is to provide electrons to photosystem I. Photosystem II catalyzes the light-
The photochemistry of photosystem II begins with the excitation of a special pair of chlorophyll molecules that are often called P680, generating P680* (Figure 22.16). P680* rapidly transfers electrons to a nearby pheophytin (designated Ph), a chlorophyll with two H+ ions in place of the central Mg2+ ion. From there, the electrons are transferred first to a tightly bound plastoquinone at site QA and then to a mobile plastoquinone at site QB. Plastoquinone is an electron carrier that closely resembles ubiquinone, a component of the mitochondrial electron-
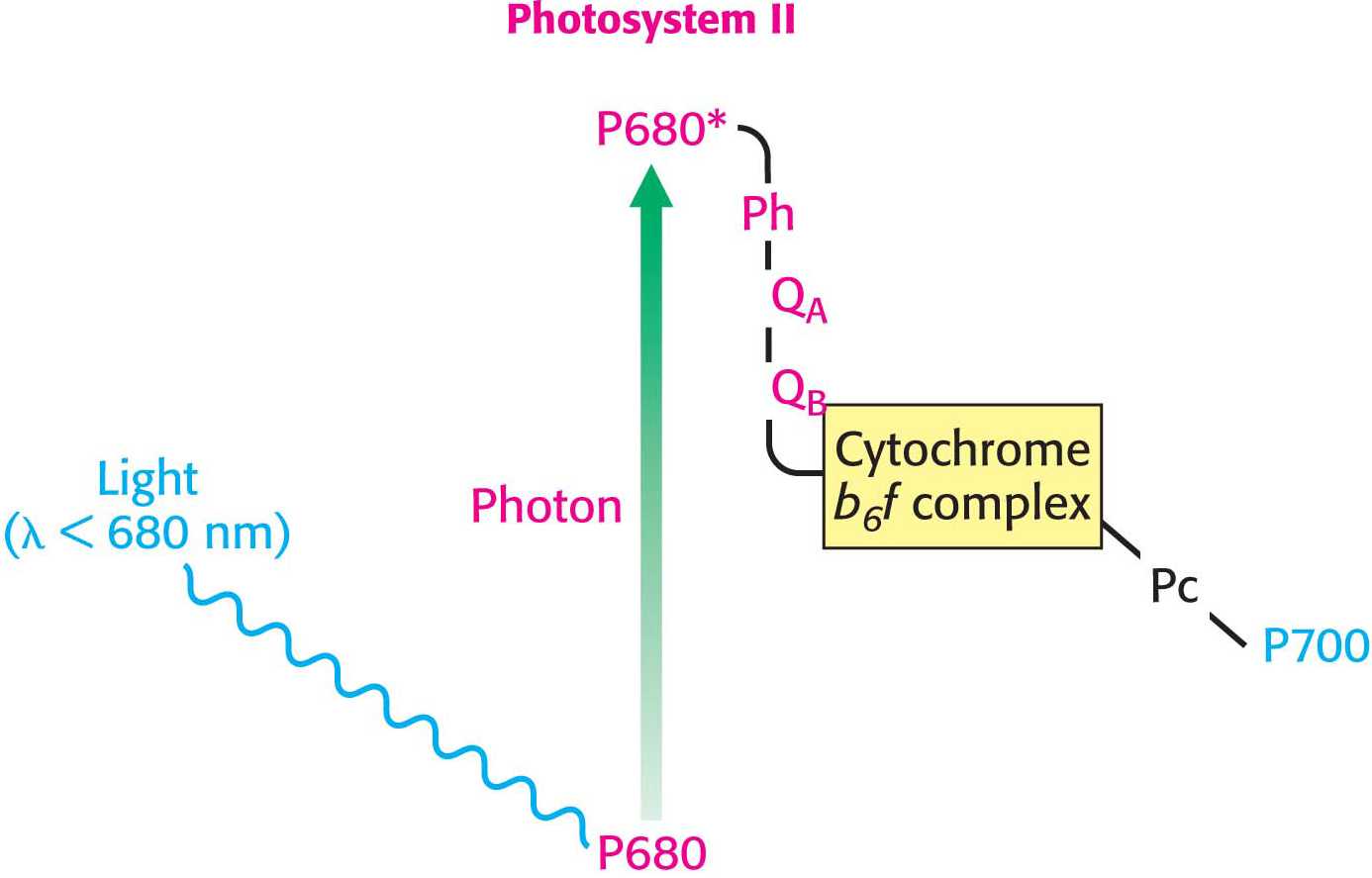
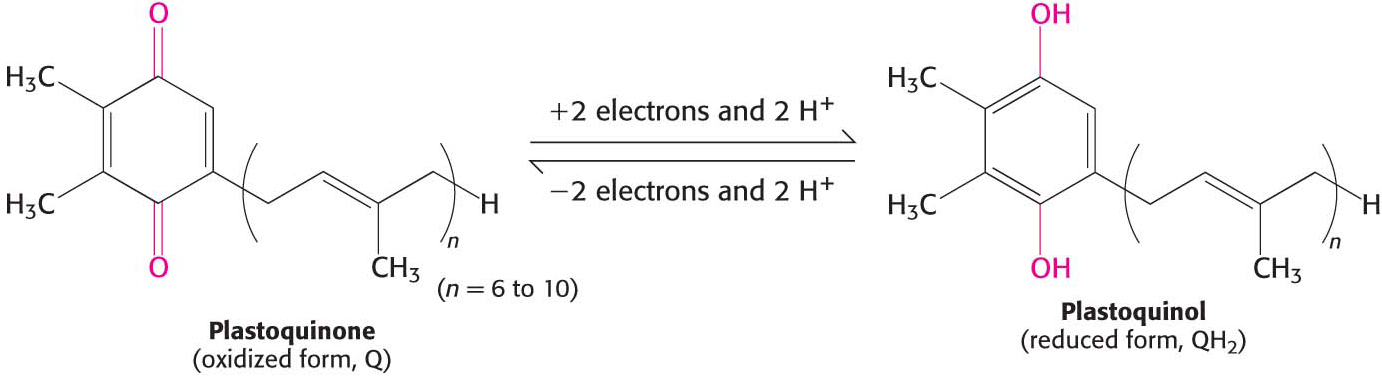
The site of quinone reduction in PS II is on the side of the stroma. Thus, the two protons that are taken up with the reduction of each molecule of plastoquinone come from the stroma, increasing the proton gradient. This gradient is further augmented by the link between photosystem II and photosystem I, the cytochrome b6 f complex.
Cytochrome b6 f Links Photosystem II to Photosystem I
The QH2 produced by photosystem II transfers its electrons to plastocyanin, which in turn donates the electrons to photosystem I, thereby replenishing the missing electrons in photosystem I. The chain starts when the electrons are transferred, one at a time from QH2, to plastocyanin (Pc), a copper-
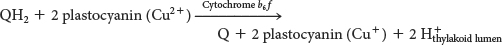
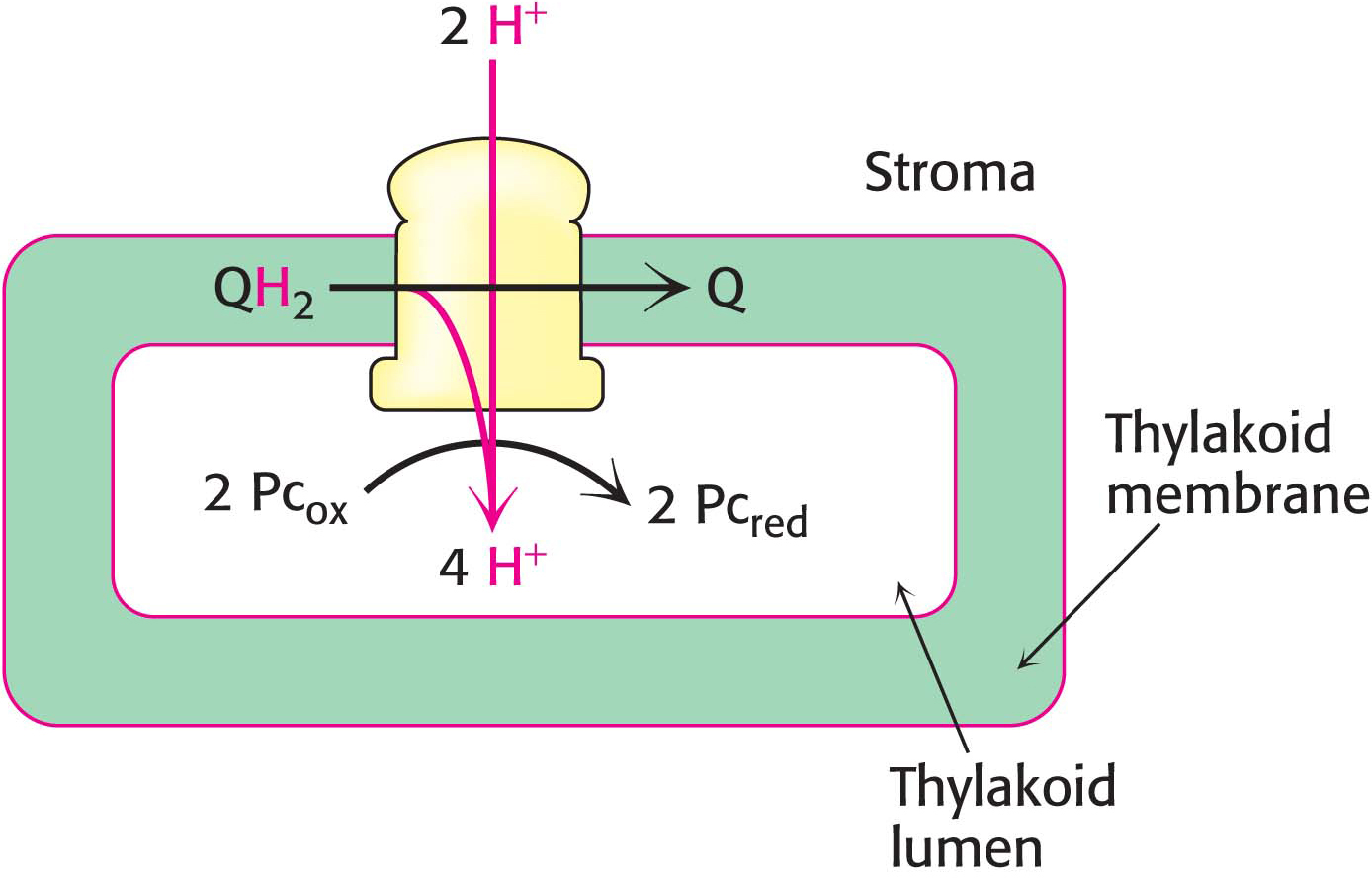
The cytochrome b6 f reaction is reminiscent of that catalyzed by ubiquinol cytochrome c oxidoreductase in oxidative phosphorylation. Indeed, most components of the cytochrome b6 f complex are similar to those of Q-
The oxidation of plastoquinol by the cytochrome b6 f complex takes place by a mechanism nearly identical with the Q cycle of the electron-
The Oxidation of Water Achieves Oxidation–Reduction Balance and Contributes Protons to the Proton Gradient
We experience the results of attaining photosynthetic redox balance with every breath that we take. The electron-
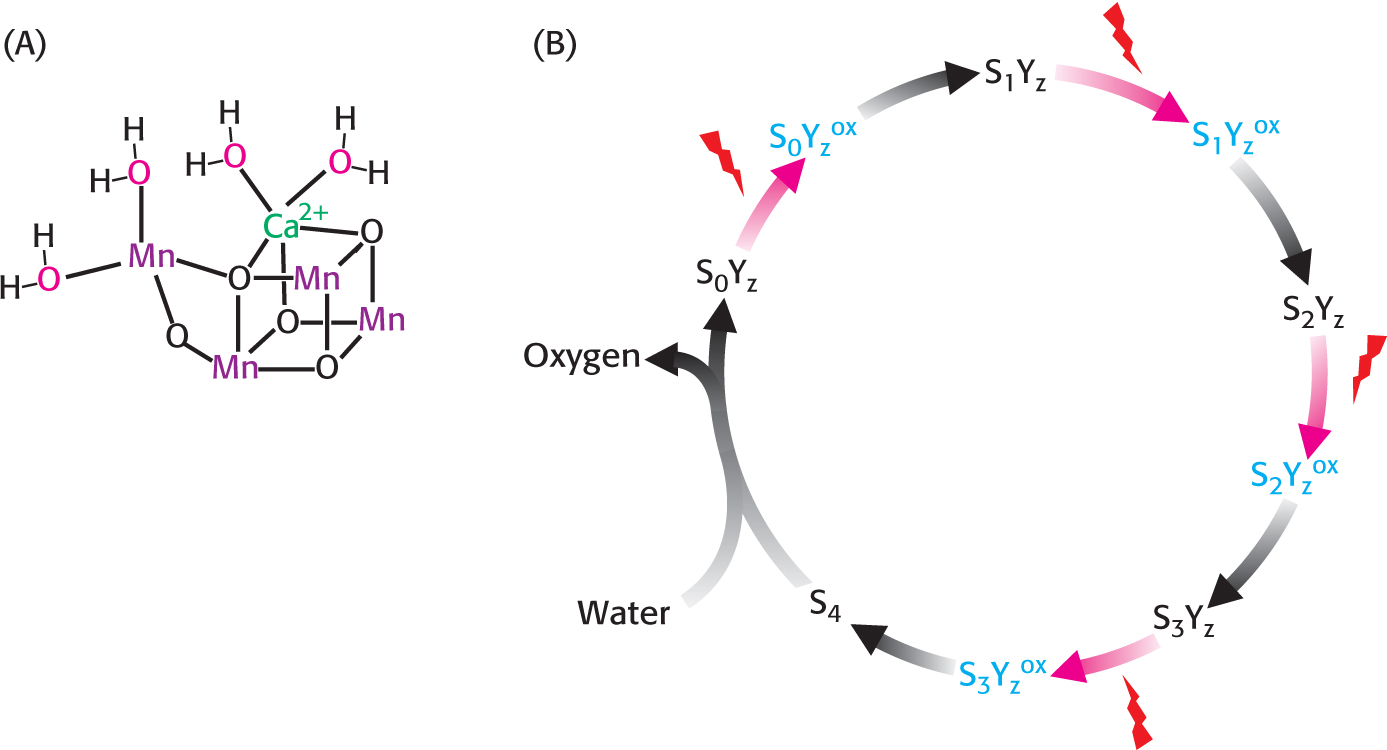
In its reduced form, the WOC oxidizes two molecules of water to form a single molecule of oxygen. Each time the absorbance of a photon powers the removal of an electron from P680, the positively charged special pair extracts an electron from a tyrosine residue of subunit D1 of the WOC, forming a tyrosine radical (Figure 22.18B). The tyrosine radical then removes an electron from a manganese ion. This process occurs four times, with the result that H2O is oxidized to generate O2 and H+:
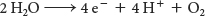

Four photons must be absorbed to extract four electrons from a water molecule. The four electrons harvested from water are used to reduce two molecules of Q to QH2. All oxygenic phototrophs, the most common type of photosynthetic organism, use the same inorganic core and protein components for capturing the energy of sunlight. A single solution to the biochemical problem of extracting electrons from water evolved billions of years ago, and has been conserved for use under a wide variety of phylogenetic and ecological circumstances. The photolysis of water by photosynthetic organisms is essentially the sole source of oxygen in the biosphere. This gas, so precious to our lives, is in essence a waste product of photosynthesis that results from the need to achieve redox balance (Figure 22.19).
The cooperation between photosystem I and photosystem II creates electron flow—
DID YOU KNOW?
“What drives life is a little electrical current, kept up by a little sunshine.”
—Albert Szent-
Nobel prize–
QUICK QUIZ
Photosystem I produces a powerful reductant, whereas photosystem II produces a powerful oxidant. Identify the reductant and oxidant and describe their roles.
Photosystem I generates ferredoxin, a powerful reductant that reduces NADP+ to NADPH, a biosynthetic reducing power. Photosystem II activates the manganese complex, a powerful oxidant capable of oxidizing water, generating O2 and electrons that will ultimately be used to reduce NADP+. In the process of oxidizing water, the manganese complex also generates protons to form the proton gradient used to generate ATP.
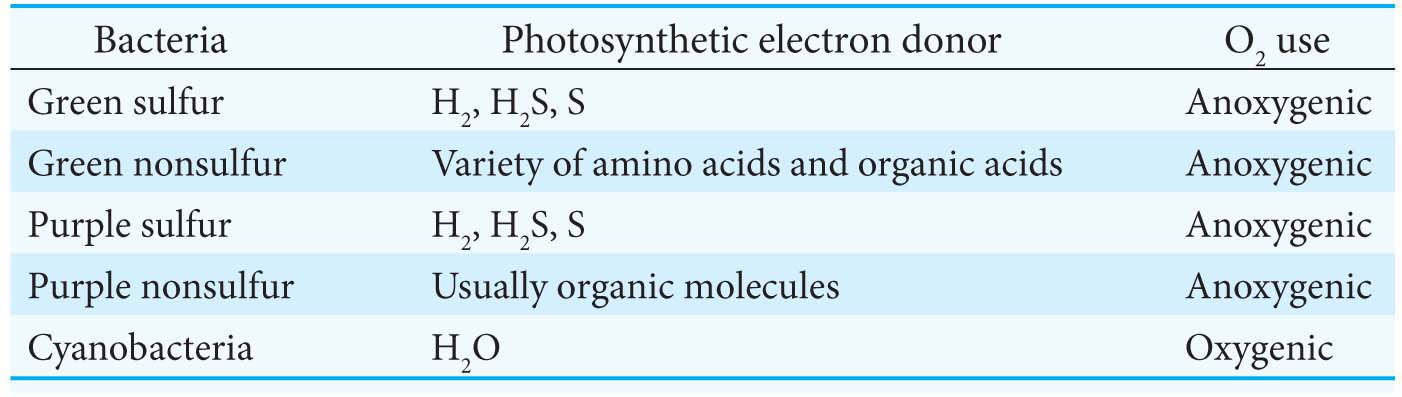
Although we have focused on the green plants, which use H2O as the electron donor in photosynthesis, Table 22.1 shows that photosynthetic bacteria display a wide variety of electron donors.