22.4 A Proton Gradient Drives ATP Synthesis
A crucial result of electron flow from water to NADP+ is the formation of a proton gradient. Such a gradient can be maintained because the thylakoid membrane is impermeable to protons. As discussed in Chapter 21, energy inherent in the proton gradient, called the proton-
The ATP Synthase of Chloroplasts Closely Resembles That of Mitochondria
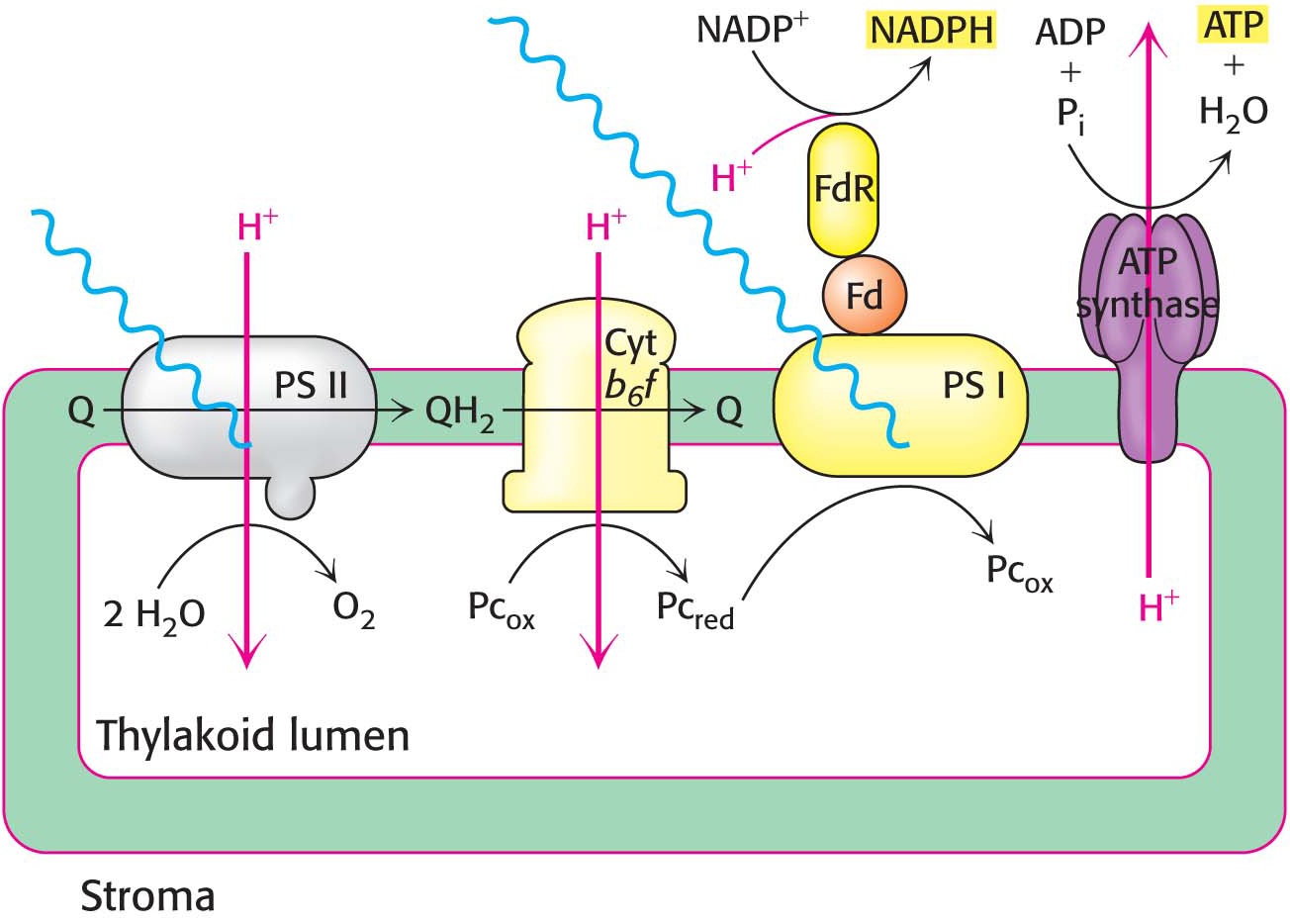
The proton-
CF0 is embedded in the thylakoid membrane. It consists of four different polypeptide chains known as I (17 kDa), II (16.5 kDa), III (8 kDa), and IV (27 kDa) with an estimated stoichiometry of 1:2:(10–
The Activity of Chloroplast ATP Synthase Is Regulated
The activity of the ATP synthase is sensitive to the redox conditions in the chloroplast. For maximal activity, a specific disulfide bond in the γ subunit must be reduced to two cysteines. The reductant is reduced thioredoxin, which is formed from ferredoxin generated in photosystem I, by ferredoxin-
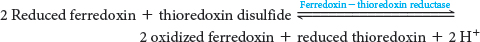
Conformational changes in the ε subunit also contribute to synthase regulation. The ε subunit appears to exist in two conformations. One conformation inhibits ATP hydrolysis by the synthase, while the other, which is generated by an increase in the proton-
Cyclic Electron Flow Through Photosystem I Leads to the Production of ATP Instead of NADPH
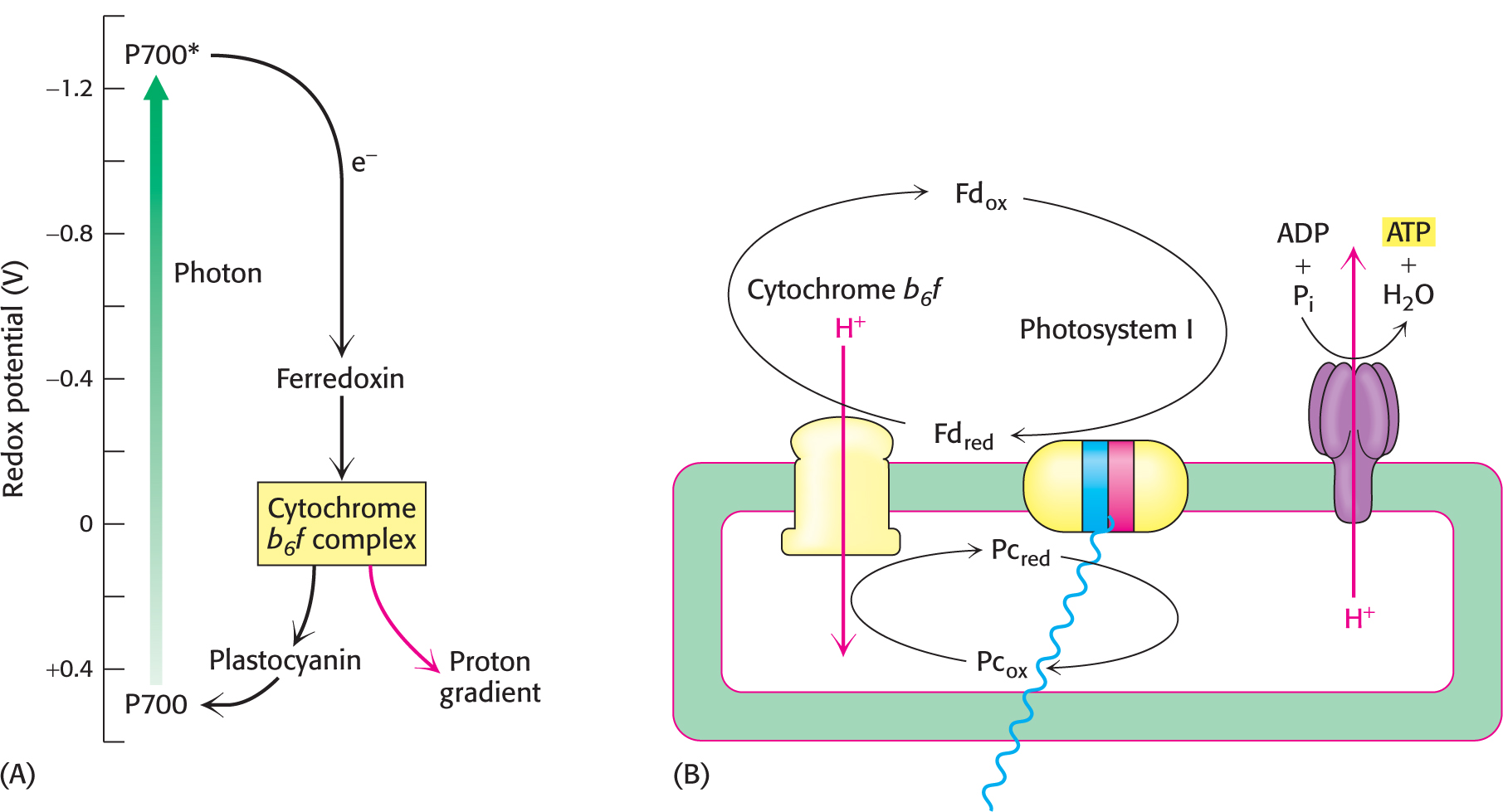
On occasion, when the ratio of NADPH to NADP+ is very high, NADP+ may not be available to accept electrons from ferredoxin. In these circumstances, specific large protein complexes allow cyclic electron flow that powers ATP synthesis, a process called cyclic photophosphorylation. In this situation, the electrons in reduced ferredoxin can be transferred back to the cytochrome b6 f complex rather than to NADP+. Each electron then flows back through the cytochrome b6 f complex to reduce plastocyanin, which can then be reoxidized by P700* to complete a cycle (Figure 22.22). The net outcome of this cyclic flow of electrons is the pumping of protons by the cytochrome b6 f complex. The resulting proton gradient then drives the synthesis of ATP. In cyclic photophosphorylation, ATP is generated independently of the formation of NADPH. Photosystem II does not participate in cyclic photophosphorylation, and so O2 is not formed from H2O.
The Absorption of Eight Photons Yields one O2, Two NADPH, and Three ATP Molecules
We can now estimate the overall stoichiometry for the light reactions. The absorption of 4 photons by photosystem II generates one molecule of O2 and releases 4 protons into the thylakoid lumen. The two molecules of plastoquinol are oxidized by the Q cycle of the cytochrome b6 f complex to release 8 protons into the lumen. The absorption of 4 additional photons by photosystem I generates four molecules of reduced plastocyanin, which subsequently reduce four molecules of ferredoxin. The four molecules of reduced ferredoxin generate two molecules of NADPH. Thus, the overall reaction is

The 12 protons released in the lumen can then flow through ATP synthase. Let us assume that there are 12 subunit III components in CF0. Twelve protons must pass through CF0 to complete one full rotation of CF1. Just as in mitochondrial ATP synthase, a single rotation generates three molecules of ATP. Given the ratio of three molecules of ATP to 12 protons, the overall reaction is
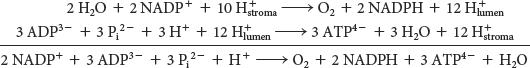
Thus, 8 photons are required to yield three molecules of ATP (2.7 photons/ATP).
Cyclic photophosphorylation is a somewhat more productive way to synthesize ATP than noncyclic photophosphorylation (the Z scheme). The absorption of 4 photons by photosystem I leads to the release of 8 protons into the lumen by the cytochrome b6 f system. These protons flow through ATP synthase to yield two molecules of ATP. Thus, every 2 photons absorbed yield one molecule of ATP. No NADPH is produced.
The Components of Photosynthesis Are Highly Organized
The complexity of photosynthesis, seen already in the elaborate interplay of multiple components, extends even to the placement of the components in the thylakoid membranes. The thylakoid membranes of most plants are differentiated into stacked (appressed) and unstacked (nonappressed) regions. Stacking increases the amount of thylakoid membrane in a given chloroplast volume. Both regions surround a common internal thylakoid lumen, but only unstacked regions make direct contact with the chloroplast stroma. Stacked and unstacked regions differ in the nature of their photosynthetic assemblies (Figure 22.23). Photosystem I and ATP synthase are located almost exclusively in unstacked regions, whereas photosystem II is present mostly in stacked regions. The cytochrome b6 f complex is found in both regions. Plastoquinone and plastocyanin are the mobile carriers of electrons between assemblies located in different regions of the thylakoid membrane. A common internal thylakoid lumen enables protons liberated by photosystem II in stacked membranes to be utilized by ATP synthase molecules that are located far away in unstacked membranes.
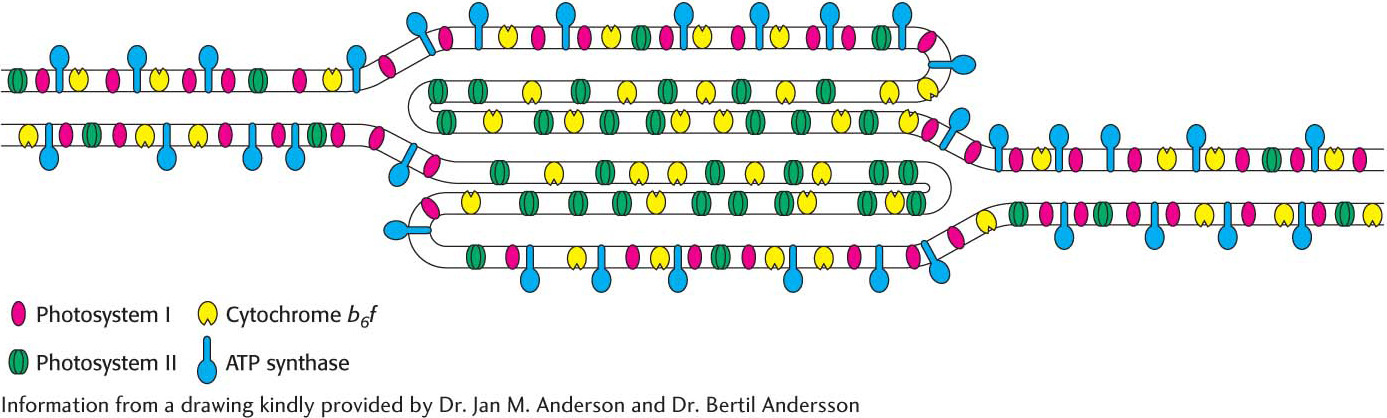
What is the functional significance of this spatial differentiation of the thylakoid membrane system? The positioning of photosystem I in the unstacked membranes gives it direct access to the stroma for the reduction of NADP+. ATP synthase, too, is located in the unstacked region to provide space for its large CF1 globule and so that it can bind stromal ADP and phosphate and release its ATP product into the stroma. The raw materials for CO2 reduction and carbohydrate synthesis are thus accessible to the stromal enzymes. The tight quarters of the appressed region pose no problem for photosystem II, which interacts with two molecules found in the thylakoid lumen—

 BIOLOGICAL INSIGHT
BIOLOGICAL INSIGHTMany Herbicides Inhibit the Light Reactions of Photosynthesis
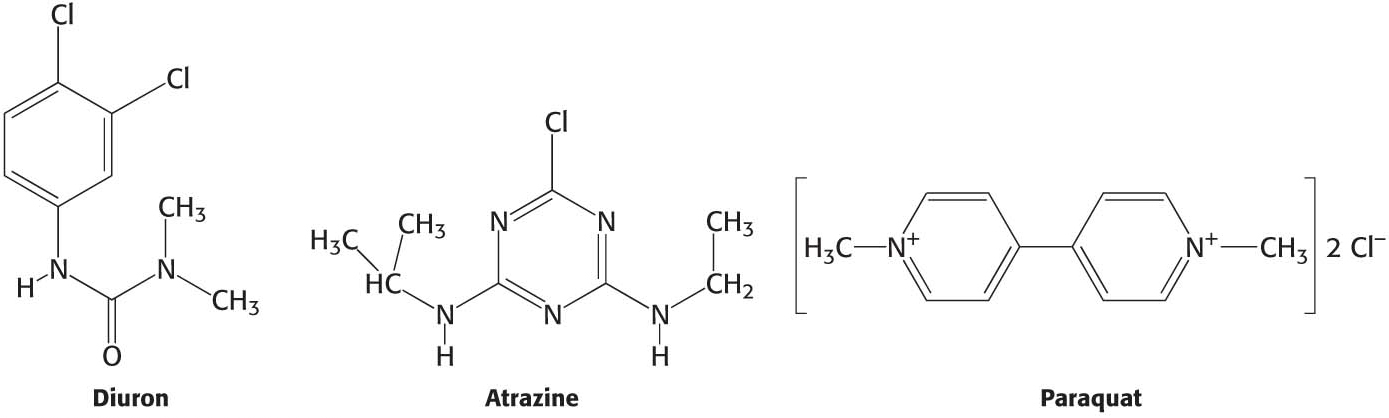
Many commercial herbicides kill weeds by interfering with the action of one of the photosystems (Figure 22.24). Inhibitors of photosystem II block electron flow, whereas inhibitors of photosystem I divert electrons from ferredoxin, the terminal part of this photosystem. Photosystem II inhibitors include urea derivatives such as diuron and triazine derivatives such as atrazine, the most widely used weed killer in the United States. These chemicals bind to a site on the D1 subunit of photosystem II and block the formation of plastoquinol (QH2).
Paraquat (1,1′-dimethyl-