PROBLEMS
Question 22.1
1. A crucial prereq. Human beings do not produce energy by photosynthesis, yet this process is critical to our survival. Explain. ✓ 2
Question 22.2
2. The accounting. What is the overall reaction of photosynthesis? ✓ 1 ✓ 3
Question 22.3
3. Like a fife and drum. Match each term with its description. ✓ 1
Light reactions Chloroplasts Reaction center Chlorophyll Light- Photosystem I Photosystem II Cytochrome b6 f complex Water- ATP synthase | Pumps protons Site of oxygen generation Primary photosynthetic pigment Transfers electrons from H2O to P700 Cellular location of photosynthesis Uses resonance energy transfer to reach the reaction center Site of photoinduced charge separation CF1-CF0 complex Generates ATP, NADPH, and O2 Generates NADPH |
Question 22.4
4. A single wavelength. Photosynthesis can be measured by measuring the rate of oxygen production. When plants are exposed to light of wavelength 680 nm, more oxygen is evolved than if the plants are exposed to light of 700 nm. Explain. ✓ 2
Question 22.5
5. Combining wavelengths. If plants described in problem 4 are illuminated by a combination of light of 680 nm and 700 nm, the oxygen production exceeds that of either wavelength alone. Explain. ✓ 2
Question 22.6
6. If a little is good. What is the advantage of having an extensive set of thylakoid membranes in the chloroplasts? ✓ 2
Question 22.7
7. Separating charges. What is the significance of photoinduced separation of charge in photosynthesis? ✓ 1
Question 22.8
8. Cooperation. Explain how light-
Question 22.9
9. One thing leads to another. Identify the ultimate electron acceptor and the ultimate electron donor in photosynthesis. What powers the electron flow between the donor and the acceptor? ✓ 3
Question 22.10
10. Neutralization compensation. In chloroplasts, a greater pH gradient across the thylakoid membrane is required to power the synthesis of ATP than is required across the mitochondrial inner membrane. Explain. ✓ 2
Question 22.11
11. Environmentally appropriate. Chlorophyll is a hydrophobic molecule. Why is this property crucial for the function of chlorophyll? ✓ 1
Question 22.12
12. Networking. Why is chlorophyll an effective light-
Question 22.13
13. Proton origins. What are the various sources of protons that contribute to the generation of a proton gradient in chloroplasts? ✓ 3
Question 22.14
14. That’s not right! Explain the defect or defects in the hypothetical scheme for the light reactions of photosynthesis depicted here.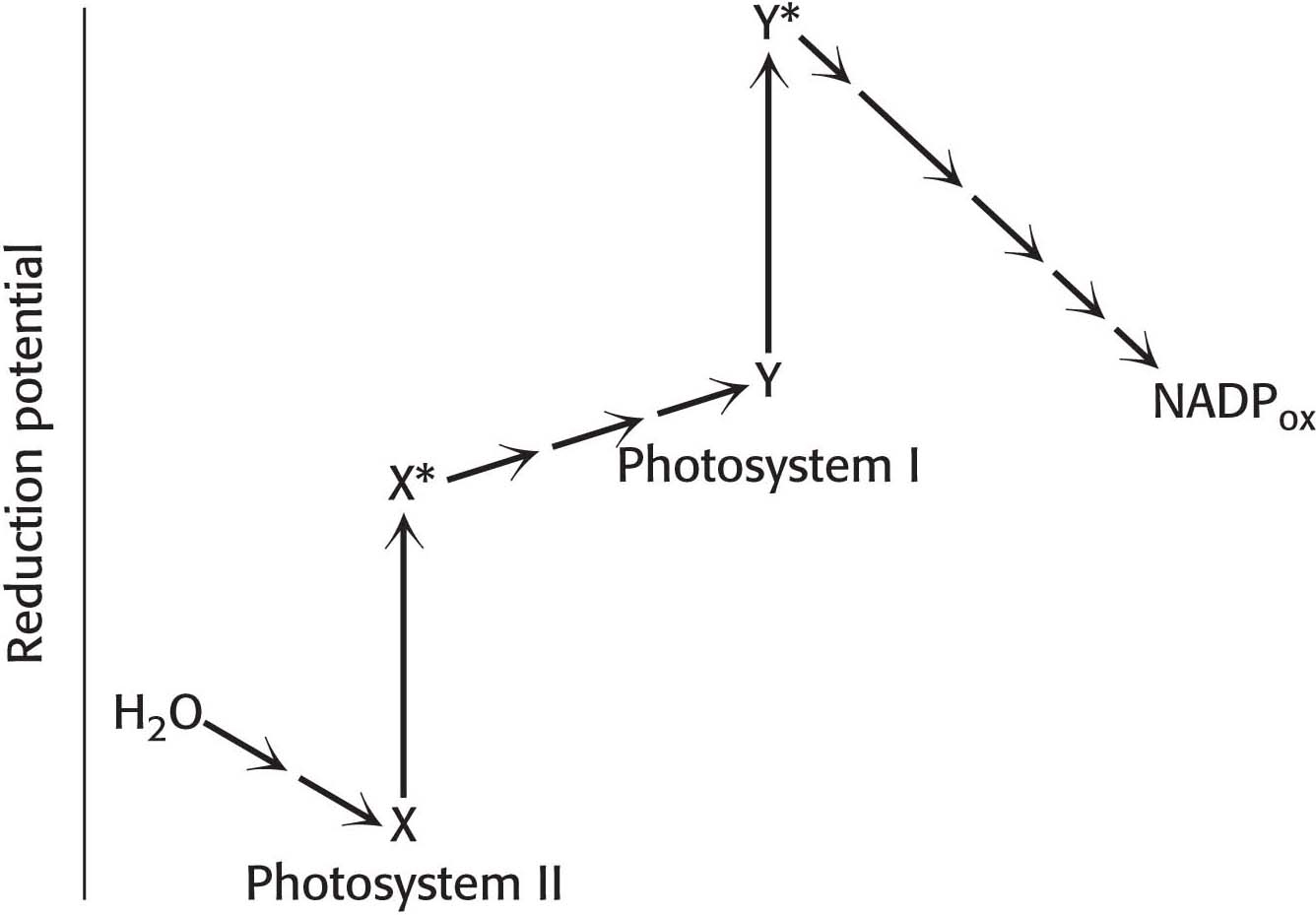
Question 22.15
15. Electron transfer. Calculate the ΔE0′ and ΔG°′ for the reduction of NADP+ by ferredoxin. Use the data given in Table 20.1. ✓ 3
Question 22.16
16. To boldly go. (a) It can be argued that, if life were to exist elsewhere in the universe, it would require some process like photosynthesis. Why is this argument reasonable? (b) If the starship Enterprise were to land on a distant planet and find no measurable oxygen in the atmosphere, could the crew conclude that photosynthesis is not taking place?
Question 22.17
17. Weed killer 1. Dichlorophenyldimethylurea (DCMU), a herbicide, interferes with photophosphorylation and O2 evolution. However, it does not block O2 evolution in the presence of an artificial electron acceptor such as ferricyanide. Propose a site for the inhibitory action of DCMU. ✓ 3
Question 22.18
18. Weed killer 2. Predict the effect of the herbicide dichlorophenyldimethylurea (DCMU) on a plant’s ability to perform cyclic photophosphorylation. ✓ 3
Question 22.19
19. Gedanken experiment. Suppose you had a suspension of chloroplasts that lacked ADP and Pi. You exposed these chloroplasts to light for a period of time, after which you plunged them into darkness while simultaneously adding ADP and Pi. To what extent, if any, would you expect ATP synthesis to take place?
Question 22.20
20. Staunching the flow. Venturicidin, an antibiotic isolated from a strain of Streptomyces, inhibits proton flow through the CF0 subunit of chloroplast ATP synthase. What would be the effect of adding venturicidin to a suspension of chloroplasts that are robustly generating oxygen? ✓ 2
Question 22.21
21. Increasing the flow. Consider again the situation described in problem 20. What could you add to the inhibited suspension of chloroplasts that would restore oxygen evolution? ✓ 2
Chapter Integration Problems
Question 22.22
22. Functional equivalents. What structural feature of mitochondria corresponds to the thylakoid membranes?
Question 22.23
23. Compare and contrast. Compare and contrast oxidative phosphorylation and photosynthesis.
Question 22.24
24. Backward. In what way is the electron transfer in ferridoxin-
Question 22.25
25. Looking for a place to rest. Albert Szent-
Data Interpretation and Chapter Integration Problem
Question 22.26
26. The same, but different. The α3β3γ complex of mitochondrial or chloroplast ATP synthase will function as an ATPase in vitro. The chloroplast enzyme (both synthase and ATPase activity) is sensitive to redox control, whereas the mitochondrial enzyme is not. To determine where the enzymes differ, a segment of the mitochondrial γ subunit was removed and replaced with the equivalent segment from the chloroplast γ subunit. The ATPase activity of the modified enzyme was then measured as a function of redox conditions. Dithioth-
(a) What is the redox regulator of the chloroplast ATP synthase in vivo? The adjoining graph shows the ATPase activity of modified and control enzymes under various redox conditions.
(b) What is the effect of increasing the reducing power of the reaction mixture for the control and the modified enzymes?
(c) What is the effect of the addition of thioredoxin? How do these results differ from those in the presence of DTT alone? Suggest a possible explanation for the difference.
(d) Did the researchers succeed in identifying the region of the γ subunit responsible for redox regulation?
(e) What is the biological rationale of regulation by high concentrations of reducing agents?
(f) What amino acids in the γ subunit are most likely affected by the reducing conditions?
(g) What experiments might confirm your answer to part f?
Challenge Problems
Question 22.27
27. Efficiency matters. What fraction of the energy of 700-
Question 22.28
28. Infrared harvest. Consider the relation between the energy of a photon and its wavelength. ✓ 1
(a) Some bacteria are able to harvest 1000-
(b) What is the maximum increase in redox potential that can be induced by a 1000-
(c) What is the minimum number of 1000-
Question 22.29
29. Hill reaction. In 1939, Robert Hill discovered that chloroplasts evolve O2 when they are illuminated in the presence of an artificial electron acceptor such as ferricyanide [Fe3+(CN)6]3−. Ferricyanide is reduced to ferrocyanide [Fe2+(CN)6]4− in this process. No NADPH or reduced plastocyanin is produced. Propose a mechanism for the Hill reaction. ✓ 3
Question 22.30
30. Energy accounts. In "Three Molecules of ATP and Two Molecules of NADPH Are Used to Bring Carbon Dioxide to the Level of a Hexose", within Section 23.1, the balance sheet for the cost of the synthesis of glucose is presented. Eighteen molecules of ATP are required. Yet, when glucose undergoes combustion in cellular respiration, 30 molecules of ATP are produced. Account for the difference. ✓ 2
Selected Readings for this chapter can be found online at www.whfreeman.com/