
23.1 The Calvin Cycle Synthesizes Hexoses from Carbon Dioxide and Water
✓ 4 Explain the function of the Calvin cycle.
The Calvin cycle takes place in the stroma of chloroplasts, the photosynthetic organelles. In this vital process, carbon dioxide gas is trapped in an organic molecule, 3-
The Calvin cycle has three stages (Figure 23.1):
The fixation of CO2 by ribulose 1,5-
bisphosphate to form two molecules of 3- phosphoglycerate. Recall that 3- phosphoglycerate is an intermediate in glycolysis and gluconeogenesis. The reduction of 3-
phosphoglycerates to form hexose sugars. The regeneration of ribulose 1,5-
bisphosphate so that more CO2 can be fixed (incorporated into an organic molecule).

Keep in mind that, owing to the stoichiometry of the conversion of six molecules of CO2 into a hexose sugar, to synthesize a glucose molecule or any other hexose sugar, the three stages must take place six times. The regeneration process is a complicated one—
Carbon Dioxide Reacts with Ribulose 1,5-bisphosphate to Form Two Molecules of 3-Phosphoglycerate
The first stage in the Calvin cycle is the fixation of CO2. This highly exergonic reaction (ΔG°′ = −51.9 kJ mol−1, or −12.4 kcal mol−1) is catalyzed by ribulose 1,5-
Carbon fixation begins with the conversion of ribulose 1,5-
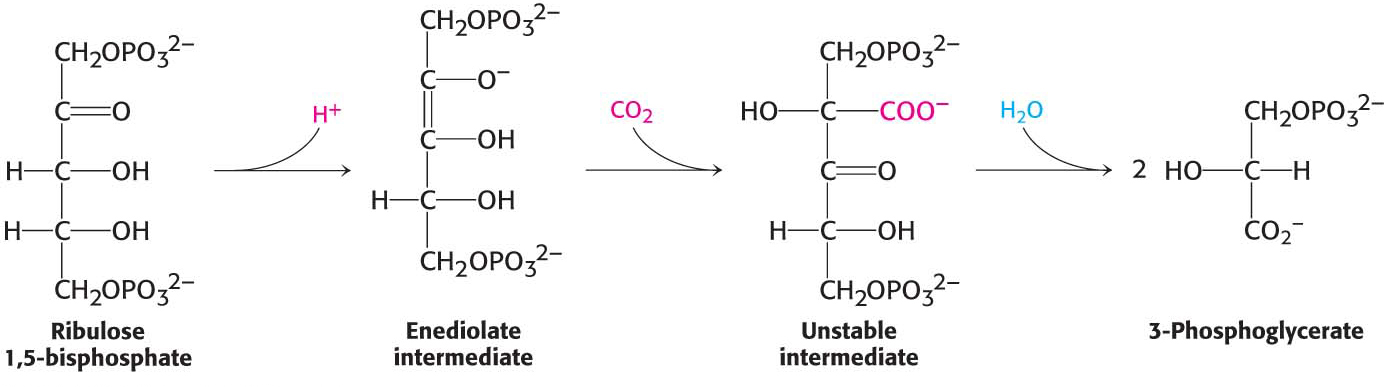
Thus, carbon dioxide, a waste product of cellular respiration, is reintroduced to the biochemical world. Plants that use only the Calvin cycle to fix carbon dioxide are called C3 plants, because the three-
Rubisco in chloroplasts consists of eight large (L, 55-
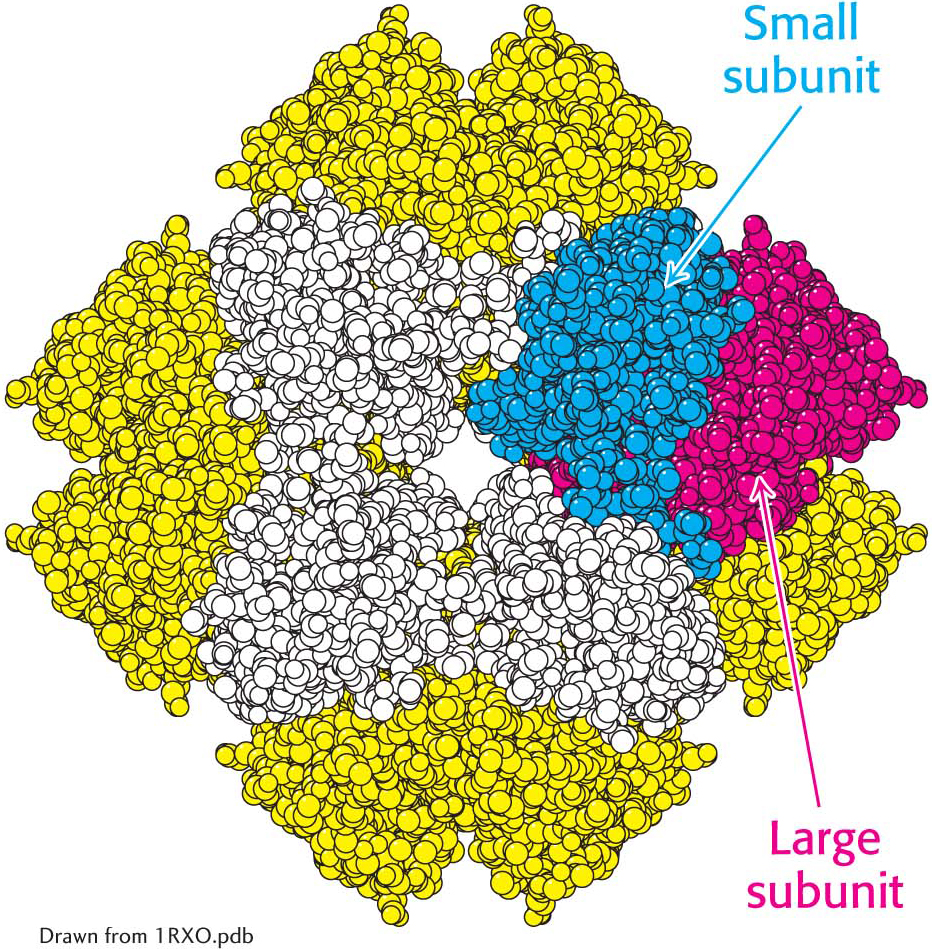
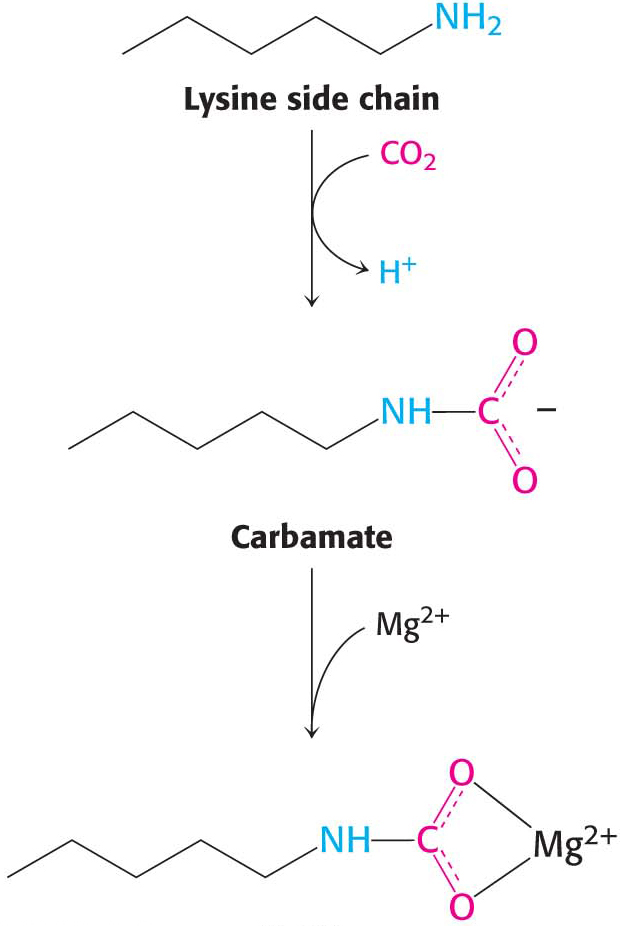
In the absence of CO2, rubisco binds to its substrate, ribulose 1,5-
Hexose Phosphates Are Made from Phosphoglycerate, and Ribulose 1,5-bisphosphate Is Regenerated
In the second stage of the Calvin cycle, the 3-

The third and final stage of the Calvin cycle is the regeneration of ribulose 1,5-

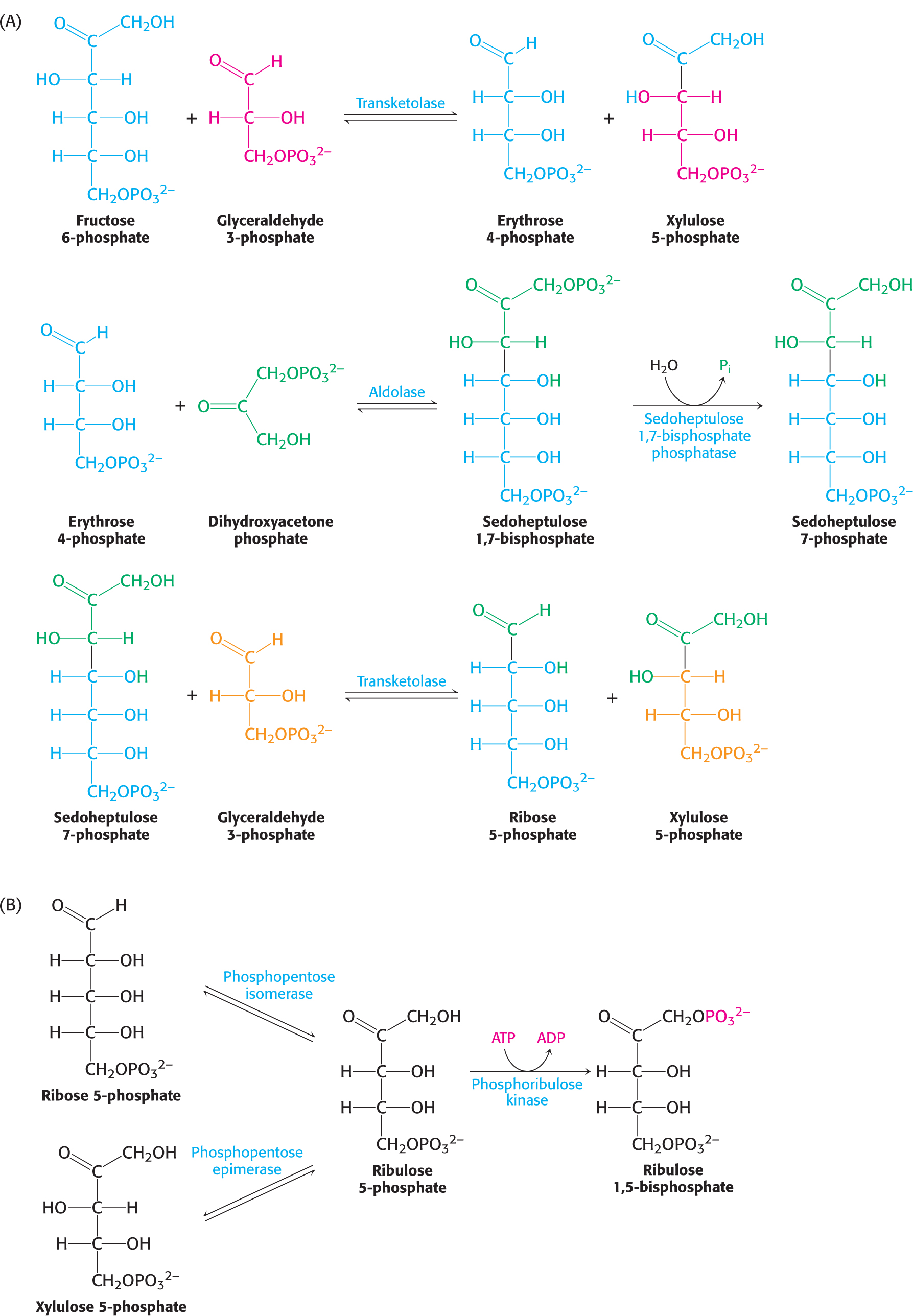
This series of reactions completes the Calvin cycle (Figure 23.5). Figure 23.5 presents the required reactions with the proper stoichiometry to convert three molecules of CO2 into one molecule of dihydroxyacetone phosphate (DHAP). However, two molecules of DHAP are required for the synthesis of a member of the hexose monophosphate pool. Consequently, the cycle as presented must take place twice to yield a hexose monophosphate. The outcome of the Calvin cycle is the generation of a hexose and the regeneration of the starting compound, ribulose 1,5-

Three Molecules of ATP and Two Molecules of NADPH Are Used to Bring Carbon Dioxide to the Level of a Hexose
What is the energy expenditure for synthesizing a hexose? Six rounds of the Calvin cycle are required, because one carbon atom is reduced in each round. Twelve molecules of ATP are expended in phosphorylating 12 molecules of 3-

Thus, 3 molecules of ATP and 2 molecules of NADPH are consumed in incorporating a single CO2 molecule into a hexose such as glucose or fructose. Biochemically, the synthesis of glucose from CO2 is energetically expensive, but the ultimate energy source—

 BIOLOGICAL INSIGHT
BIOLOGICAL INSIGHTA Volcanic Eruption Can Affect Photosynthesis Worldwide
On June 12, 1991, Mount Pinatubo, a volcano on the island of Luzon in the Philippines, erupted (Figure 23.6). The eruption, which was the largest on Earth in a hundred years, killed more than 700 people. The eruption also spewed approximately 15 million tons of sulfur dioxide (SO2) into the atmosphere in the form of sulfate aerosol, encircling Earth in a massive aerosol blanket in about 3 weeks. This blanket led to a decrease in total sunlight striking Earth (direct sunlight plus diffuse sunlight) but increased the proportion of diffuse sunlight.
At the same time as the sulfate aerosol was enveloping the planet, the rate of growth of atmospheric CO2 declined sharply. Moreover, an experimental station in the northeastern United States reported that noontime photosynthesis increased by almost 25%. Could these events have been related?
If the increase in photosynthesis observed in the northeastern United States were happening globally, as might have been expected, then more CO2 would have been removed from the atmosphere by rubisco, accounting for the decrease in the rate of growth of CO2. But why did it happen? Although still not firmly established, the diffuse light caused by the sulfate aerosol may have actually increased the amount of photosynthesis taking place. Figure 23.7 shows that the rate of photosynthesis reaches a limiting value when light intensity is increased. Consequently, in bright direct sunlight, the top leaves of a forest may be receiving more light than they can biochemically use. However, in direct sunlight, the lower leaves in the shadows of the upper leaves will be light deprived. Diffuse light, on the other hand, will penetrate more deeply into the forest. More leaves will receive light, more rubisco will be active, and more CO2 will be converted into sugar.

Starch and Sucrose Are the Major Carbohydrate Stores in Plants
What are the fates of the carbon atoms fixed and processed by the enzymes of the Calvin cycle? These molecules are used in a variety of ways, but there are two prominent fates: the synthesis of starch and sucrose, storage forms of carbohydrates. Starch, like its animal counterpart glycogen, is a polymer of glucose residues. Starch comes in two forms: amylose, which consists of glucose molecules joined together by α-1,4 linkages only; and amylopectin, which is branched because of the presence of α-1,6 linkages. Starch is synthesized and stored in chloroplasts.
In contrast, sucrose (common table sugar), a disaccharide, is synthesized in the cytoplasm. Plants lack the ability to transport hexose phosphates across the chloroplast membrane, but an abundant triose phosphatephosphate antiporter mediates the transport of triose phosphates from chloroplasts to the cytoplasm in exchange for phosphate. Fructose 6-


QUICK QUIZ 1
Why is the Calvin cycle crucial to the functioning of all life forms?
The Calvin cycle is the primary means of converting gaseous CO2 into organic matter—
 BIOLOGICAL INSIGHT
BIOLOGICAL INSIGHTWhy Bread Becomes Stale: The Role of Starch
Bread staling (the process of becoming stale) is a complex process in which all of the components of bread take part—
As the bread cools after it has been removed from the oven (Figure 23.10), amylose molecules separate from the amorphous starch structure and begin to associate with one another; that is, the amylose crystallizes. This crystallization takes place most prominently on the surface of the bread, where it is facilitated by the evaporation of surface water. This crystallization contributes to the crispness of the surface.

Meanwhile, inside the bread, crystallization of the amylopectin of the crumb is slower, possibly because of its branched structure, and continues for several days. Water bound to the amylopectin is released as the crystals form, which leads to a deterioration of the texture and taste of the crumb. Water is still present, but no longer in association with the amylopectin molecules. In fact, staling can be reversed at this stage if the bread is reheated to rehydrate the internal amylose and amylopectin crystals. However, if the bread is left uncovered, water will move to the surface, destroying the crispness of the bread and subsequently evaporating. Further evaporation leads to further crystallization, and, eventually, we are left with a loaf of bread more suitable for use as a doorstop.
