
Glycogen Degradation


Glycogen is a very large, branched polymer of glucose residues (Figure 24.1). Most of the glucose residues in glycogen are linked by α-1,4-
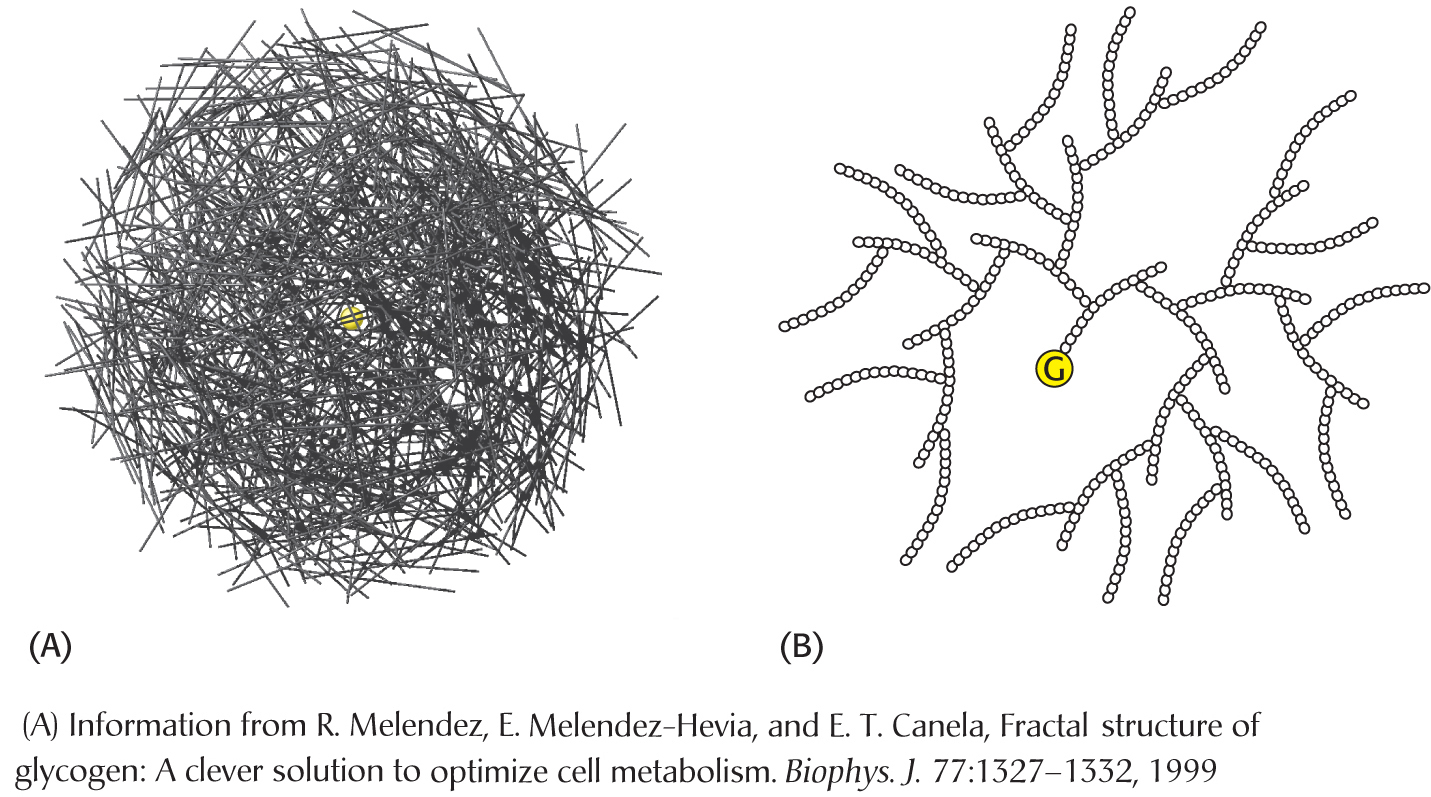
Glycogen is present in bacteria, archae, and eukaryotes. Recall that plants store glucose as starch, a similar biomolecule. Thus, storing energy as glucose polymers is common to all forms of life. In humans, most tissues have some glycogen, but the two major sites of glycogen storage are the liver and skeletal muscle. The concentration of glycogen is higher in the liver than in muscle (10% versus 2% by weight), but more glycogen is stored in skeletal muscle overall because there is more skeletal muscle in the body than there is liver tissue. Glycogen is present in the cytoplasm in the form of granules ranging in diameter from 10 to 40 nm, containing about 55,000 glucose molecules. In the liver, glycogen synthesis and degradation are regulated to maintain the concentration of glucose in the blood required to meet the needs of the organism as a whole. The glucose is parceled out from the liver during a nocturnal fast, maintaining brain function throughout the night. In contrast, in muscle, these processes are regulated to meet the energy needs of the muscle itself. Glycogen breakdown takes place to fuel the ATP needs of muscle contraction. The depletion of muscle glycogen is thought to be a major component of exhaustion—