
24.2 Phosphorylase Is Regulated by Allosteric Interactions and Reversible Phosphorylation
✓ 2 Explain the regulation of glycogen breakdown.
DID YOU KNOW?
Isozymes, or isoenzymes, are enzymes that are encoded by different genes yet catalyze the same reaction. Usually, isozymes display different kinetic parameters or regulatory properties.
Glycogen degradation is precisely controlled by multiple interlocking mechanisms. The focus of this control is the enzyme glycogen phosphorylase. Phosphorylase is regulated by several allosteric effectors that signal the energy state of the cell, as well as by reversible phosphorylation, which is responsive to hormones such as epinephrine, glucagon, and insulin. We will examine the differences in the control of two isozymic forms of glycogen phosphorylase: one specific to liver and one specific to skeletal muscle. These differences are due to the fact that the liver maintains glucose homeostasis of the organism as a whole, whereas the muscle uses glucose to produce energy for itself.
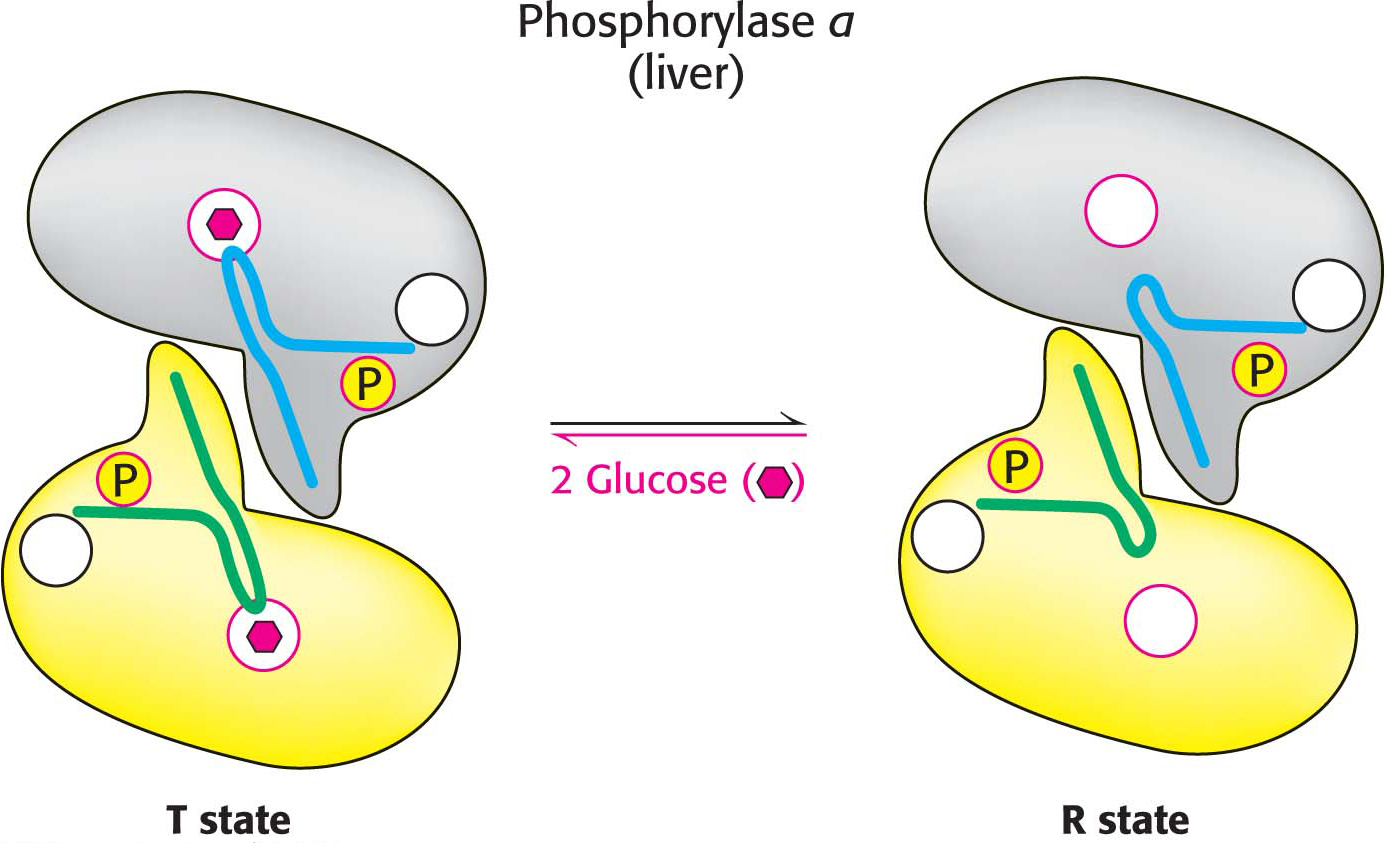
Liver Phosphorylase Produces Glucose for Use by Other Tissues
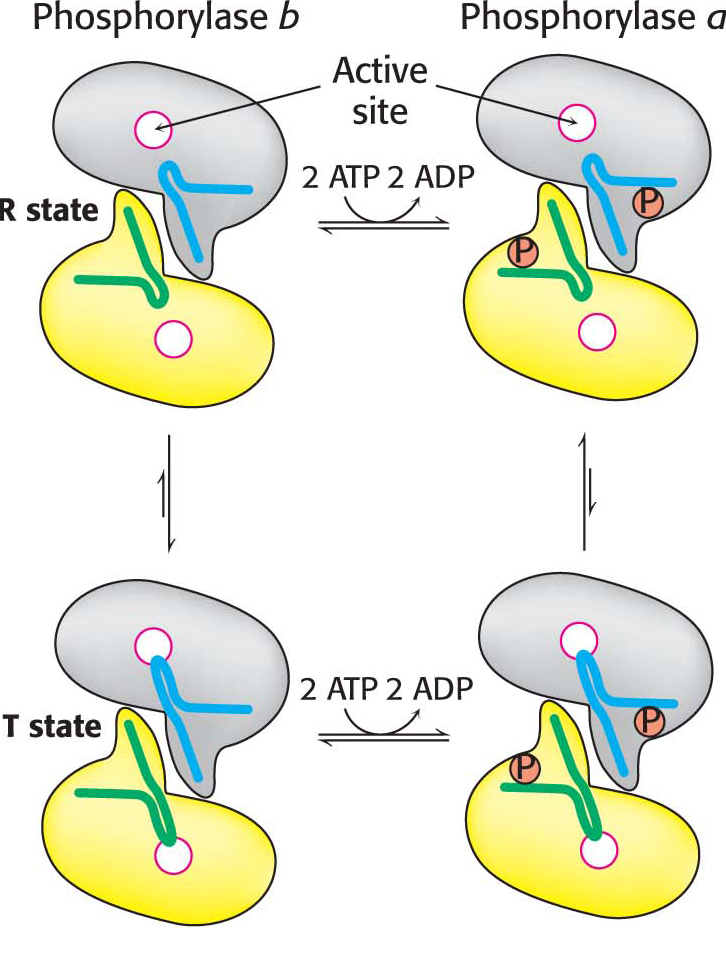
The dimeric phosphorylase exists in two interconvertible forms: a usually active phosphorylase a and a usually inactive phosphorylase b (Figure 24.5). Each of these two forms exists in equilibrium between an active relaxed (R) state and a much less active tense (T) state, but the equilibrium for phosphorylase a favors the active R state, whereas the equilibrium for phosphorylase b favors the less active T state (Figure 24.6). The role of glycogen degradation in the liver is to form glucose for export to other tissues when the blood-
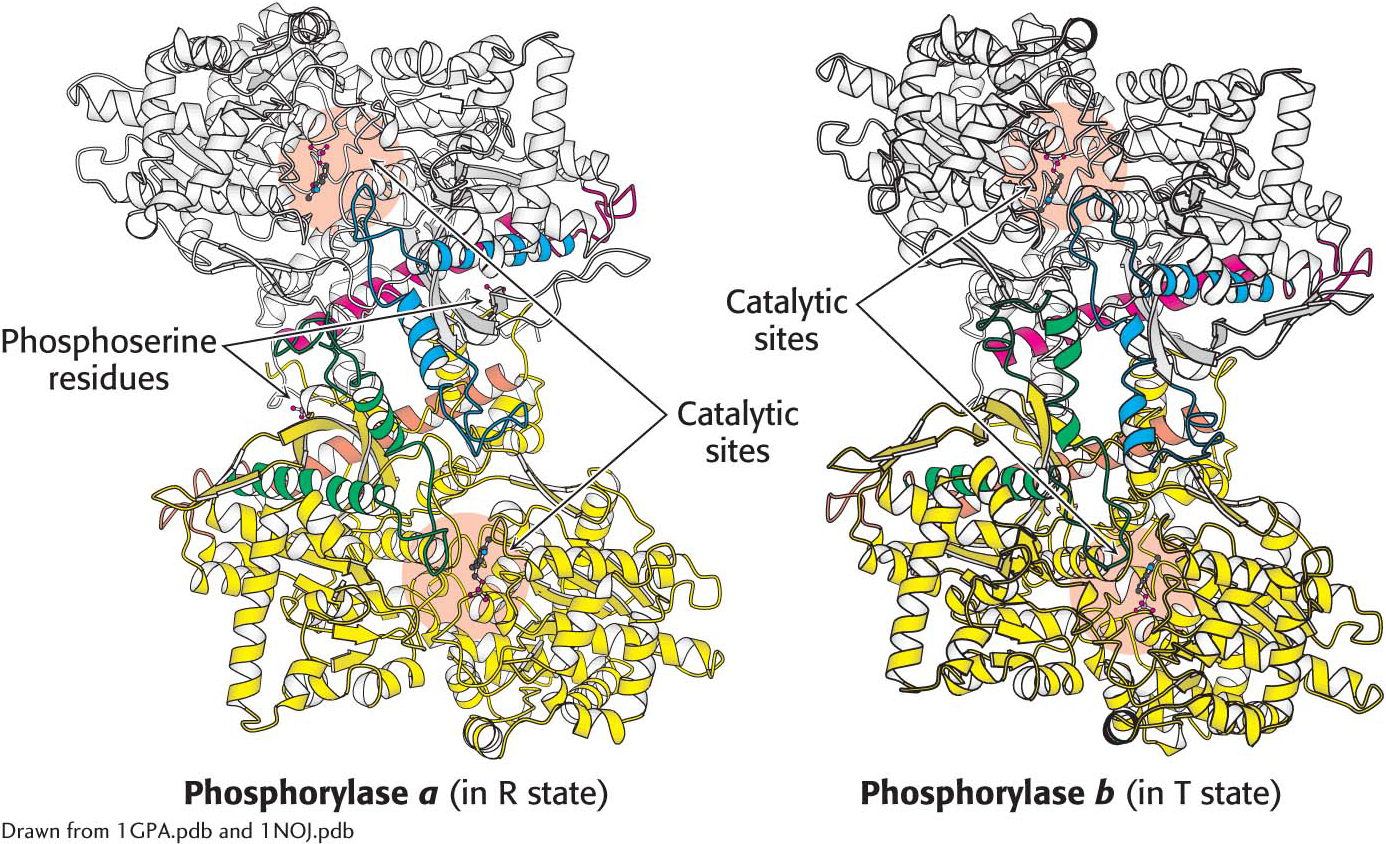
 Structures of phosphorylase a and phosphorylase b. Phosphorylase a is phosphorylated on serine 14 of each subunit. This modification favors the structure of the more active R state. One subunit is shown in white, with helices and loops important for regulation shown in blue and red. The other subunit is shown in yellow, with the regulatory structures shown in orange and green. phosphorylase b is not phosphorylated and exists predominantly in the T state. Notice that the catalytic sites are partly occluded in the T state.
Structures of phosphorylase a and phosphorylase b. Phosphorylase a is phosphorylated on serine 14 of each subunit. This modification favors the structure of the more active R state. One subunit is shown in white, with helices and loops important for regulation shown in blue and red. The other subunit is shown in yellow, with the regulatory structures shown in orange and green. phosphorylase b is not phosphorylated and exists predominantly in the T state. Notice that the catalytic sites are partly occluded in the T state.
Muscle Phosphorylase Is Regulated by the Intracellular Energy Charge
In contrast to the liver isozyme, the default state of muscle phosphorylase is the b form, owing to the fact that, for muscle, phosphorylase needs to be active primarily during muscle contraction. Muscle phosphorylase b is activated by the presence of high concentrations of AMP, which binds to a nucleotide-
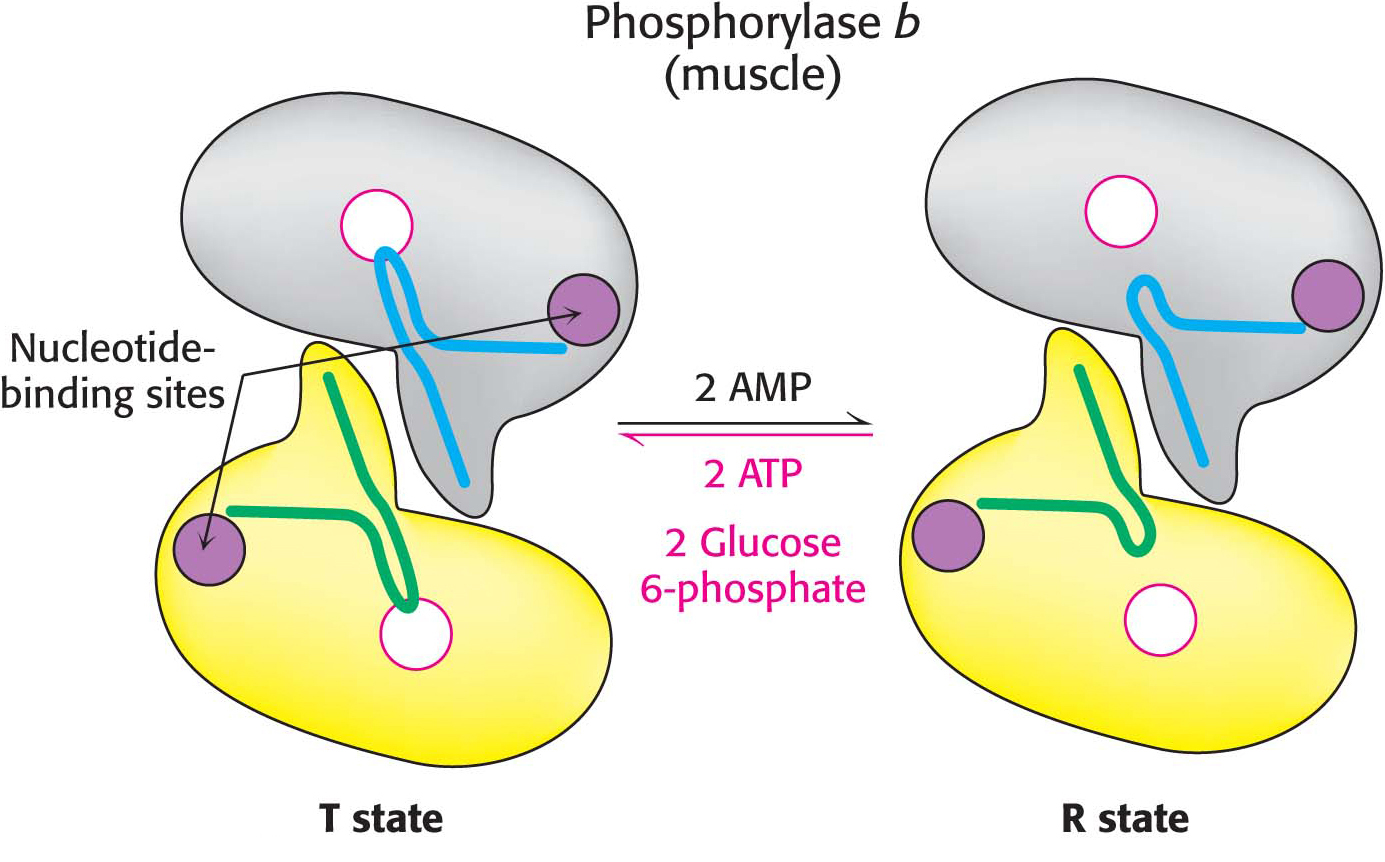
Unlike the enzyme in muscle, the liver phosphorylase is insensitive to regulation by AMP because the liver does not undergo the dramatic changes in energy charge seen in a contracting muscle. We see here a clear example of the use of isozymes to establish the tissue-
Biochemical Characteristics of Muscle Fiber Types Differ
Not only do the biochemical needs of liver and muscle differ with respect to glycogen metabolism, but the biochemical needs of different muscle fiber types also vary. Skeletal muscle consists of three different fiber types. Type I or slow-
Phosphorylation Promotes the Conversion of Phosphorylase b to Phosphorylase a
In both liver and muscle, phosphorylase b is converted into phosphorylase a by the phosphorylation of a single serine residue (serine 14) in each subunit. This conversion is initiated by hormones. Low blood-
QUICK QUIZ 2
Compare the allosteric regulation of phosphorylase in the liver and in muscle, and explain the significance of the difference.
In muscle, the b form of phosphorylase is activated by AMP. In the liver, the a form is inhibited by glucose. The difference corresponds to the difference in the metabolic role of glycogen in each tissue. Muscle uses glycogen as a fuel for contraction, whereas the liver uses glycogen to maintain proper blood-
Comparison of the structures of phosphorylase a in the R state and phosphorylase b in the T state reveals that subtle structural changes at the subunit interfaces are transmitted to the active sites (Figure 24.5). The transition from the T state (the prevalent state of phosphorylase b) to the R state (the prevalent state of phosphorylase a) is associated with structural changes in α helices that move a loop out of the active site of each subunit. Thus, the T state is less active because the catalytic site is partly blocked. In the R state, the catalytic site is more accessible and a binding site for orthophosphate is well organized.
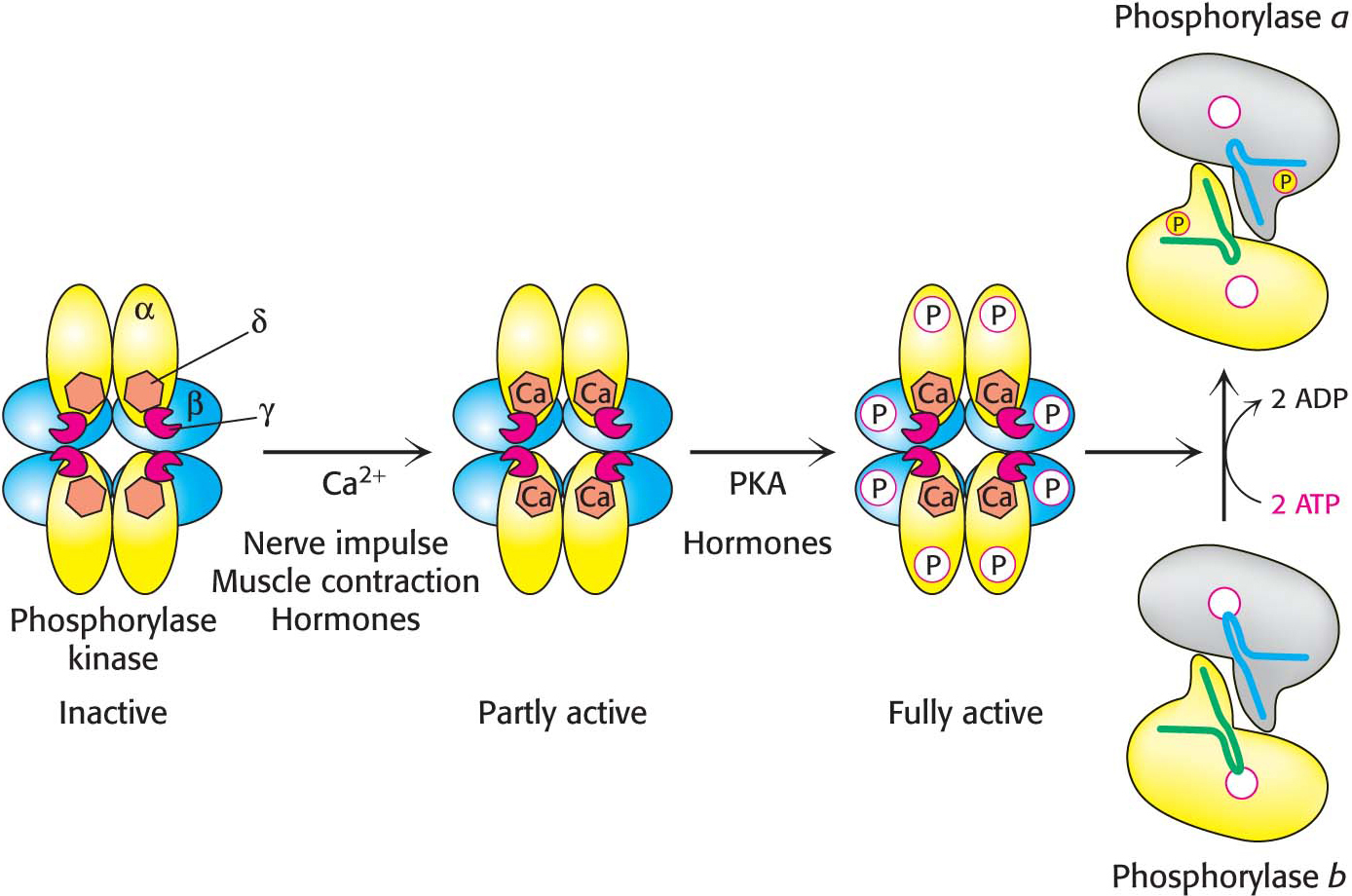
Phosphorylase Kinase Is Activated by Phosphorylation and Calcium Ions
Phosphorylase kinase activates phosphorylase b by attaching a phosphoryl group. The subunit composition of phosphorylase kinase in skeletal muscle is (αβγδ)4, and the mass of this very large protein is 1300 kDa. The enzyme consists of two (αβγδ)2 lobes that are joined by a β4 bridge that is the core of the enzyme and serves as a scaffold for the remaining subunits. The γ subunit contains the active site, while all of the remaining subunits (≈90% by mass) play regulatory roles. The δ subunit is the calcium-
Activation of phosphorylase kinase is initiated when Ca2+ binds to the δ subunit. This mode of activation of the kinase is especially noteworthy in muscle, where contraction is triggered by the release of Ca2+ from the sarcoplasmic reticulum (Figure 24.9). Maximal activation is achieved with the phosphorylation of the β and α subunits of the Ca2+-bound kinase. The stimulation of phosphorylase kinase is one step in a signal-
 CLINICAL INSIGHT
CLINICAL INSIGHTHers Disease Is Due to a Phosphorylase Deficiency
Hers disease, a hereditary disorder, is a glycogen-