24.3 Epinephrine and Glucagon Signal the Need for Glycogen Breakdown
Protein kinase A activates phosphorylase kinase, which in turn activates glycogen phosphorylase. How is protein kinase A activated? What is the signal that ultimately triggers an increase in glycogen breakdown?
G Proteins Transmit the Signal for the Initiation of Glycogen Breakdown
As already mentioned, glucagon and epinephrine trigger the breakdown of glycogen. Muscular activity or its anticipation leads to the release of epinephrine, which markedly stimulates glycogen breakdown in muscle and, to a lesser extent, in the liver. The liver is more responsive to glucagon, which signifies the starved state (Figure 24.10).
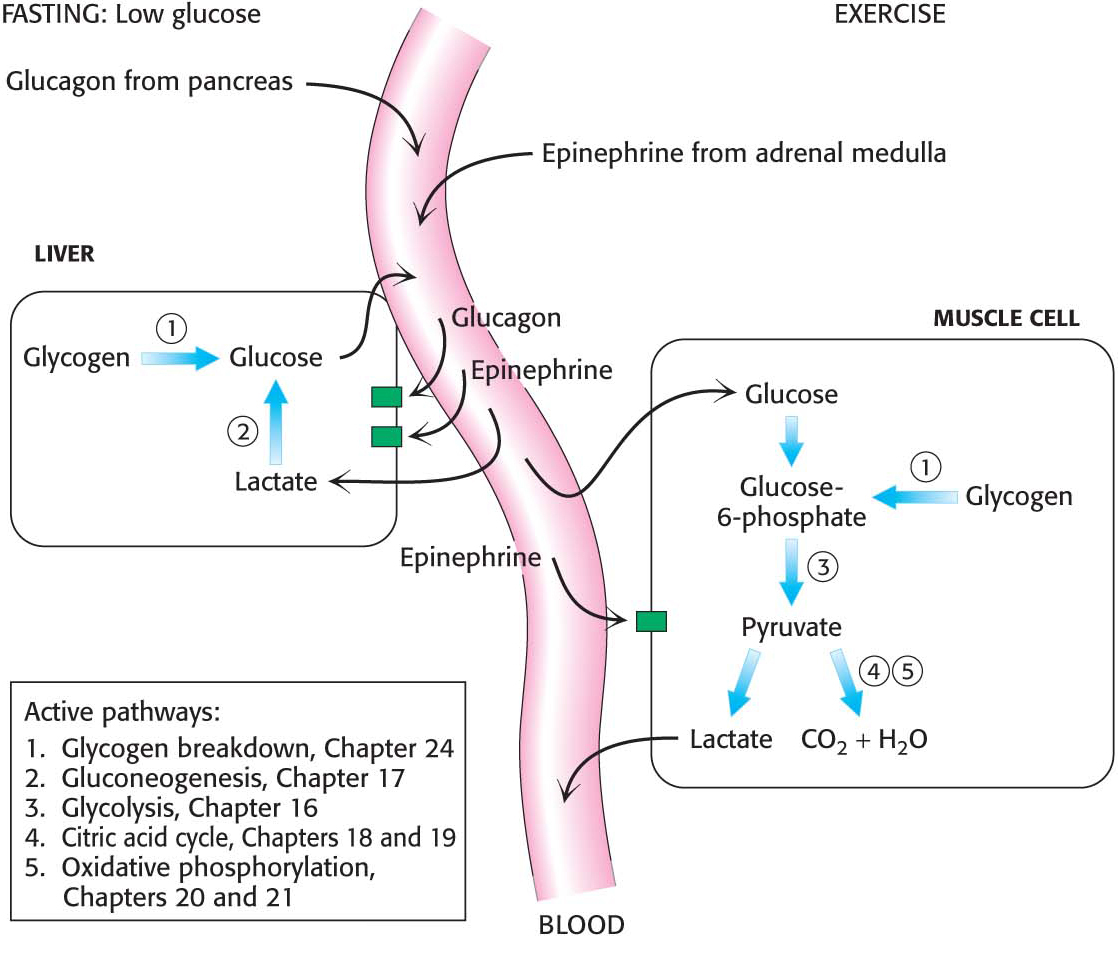
How do hormones trigger the breakdown of glycogen? They initiate a cyclic AMP (cAMP) signal-
The signal molecules epinephrine and glucagon bind to specific seven-
transmembrane (7TM) receptors in the plasma membranes of target cells. Epinephrine binds to the β-adrenergic receptor in muscle, whereas glucagon binds to the glucagon receptor in the liver. These binding events activate the Gas protein. The GTP-
bound subunit of Gas activates the transmembrane protein adenylate cyclase. This enzyme catalyzes the formation of the second messenger cAMP from ATP. The elevated cytoplasmic concentration of cAMP activates protein kinase A.
Protein kinase A phosphorylates phosphorylase kinase, which subsequently activates glycogen phosphorylase.
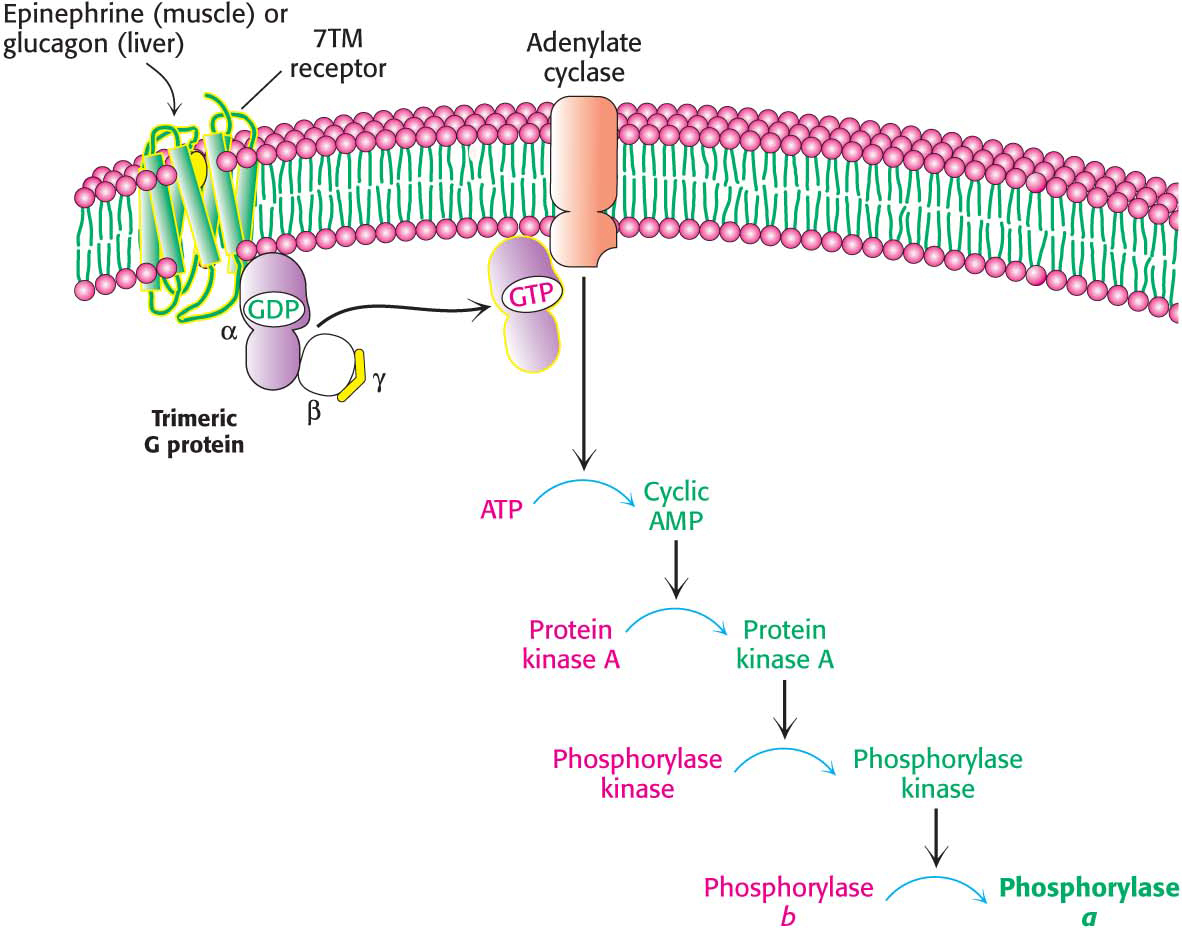
The cAMP cascade highly amplifies the effects of hormones. The binding of a small number of hormone molecules to cell-
The signal-
Glycogen Breakdown Must Be Rapidly Turned Off When Necessary
There must be a rapid way to shut down glycogen breakdown to prevent the wasteful depletion of glycogen after energy needs have been met. When glucose needs have been satisfied, phosphorylase kinase and glycogen phosphorylase are dephosphorylated and inactivated. Simultaneously, glycogen synthesis is activated (Chapter 25).
The signal-
 BIOLOGICAL INSIGHT
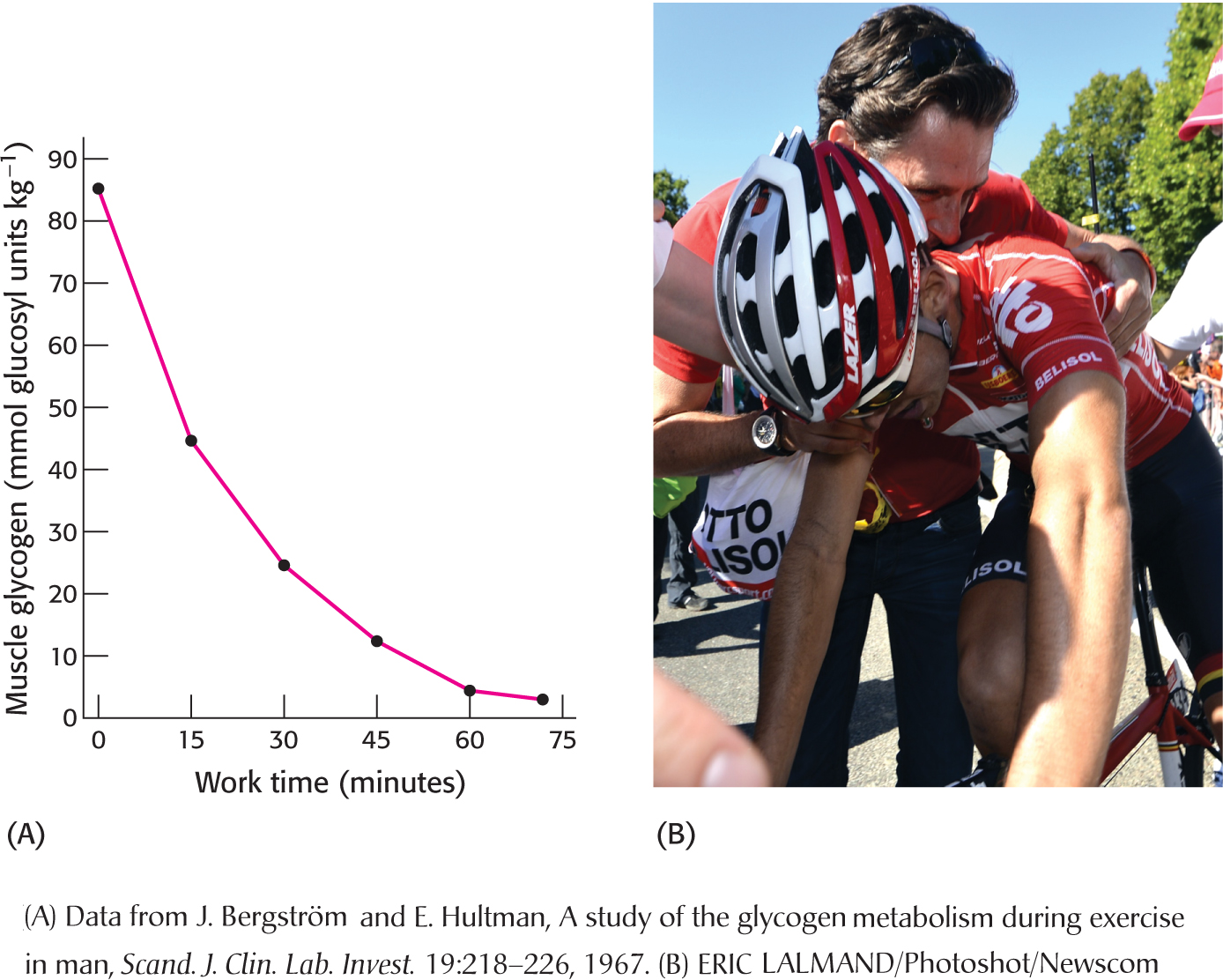
BIOLOGICAL INSIGHTGlycogen Depletion Coincides with the Onset of Fatigue
Although most of us have experienced fatigue, it is a multifaceted and difficult condition to define. There are metabolic, neurological, and psychological components to fatigue, but a common definition is simply the inability to maintain the required energy output. Figure 24.12 A shows the decrease in glycogen content of the vastus lateralis muscle (a component of the quadriceps) of cyclists who were exercising at 80% of their maximum workload as a function of time. Muscle glycogen phosphorylase was activated through allosteric mechanisms and by hormonally induced covalent modification to such an extent that, after 75 minutes, all of the glycogen in the vastus lateralis muscle was consumed. Moreover, glycogen depletion coincided with the onset of fatigue, or the inability to maintain the required effort. In other words, the cyclists “hit the wall” or “bonked” (Figure 24.12B). However, these experiments do not show that glycogen depletion causes fatigue; correlation does not mean causation. Some experiments suggest that the increase in ADP concentration resulting from glycogen depletion may be a more direct cause of the fatigue. It is fascinating that an explanation for such a common feeling still eludes scientists and demonstrates once again the scope of how little we know and the opportunities for more exciting research.