
25.1 Glycogen Is Synthesized and Degraded by Different Pathways
✓ 3 Describe the steps of glycogen synthesis, and identify the enzymes required.
✓ 4 Explain the regulation of glycogen synthesis.
A common theme for the biosynthetic pathways that we will encounter in our study of biochemistry is the requirement for an activated precursor. This axiom holds true for glycogen synthesis. Glycogen is synthesized by a pathway that utilizes uridine diphosphate glucose (UDP-

UDP-Glucose Is an Activated Form of Glucose
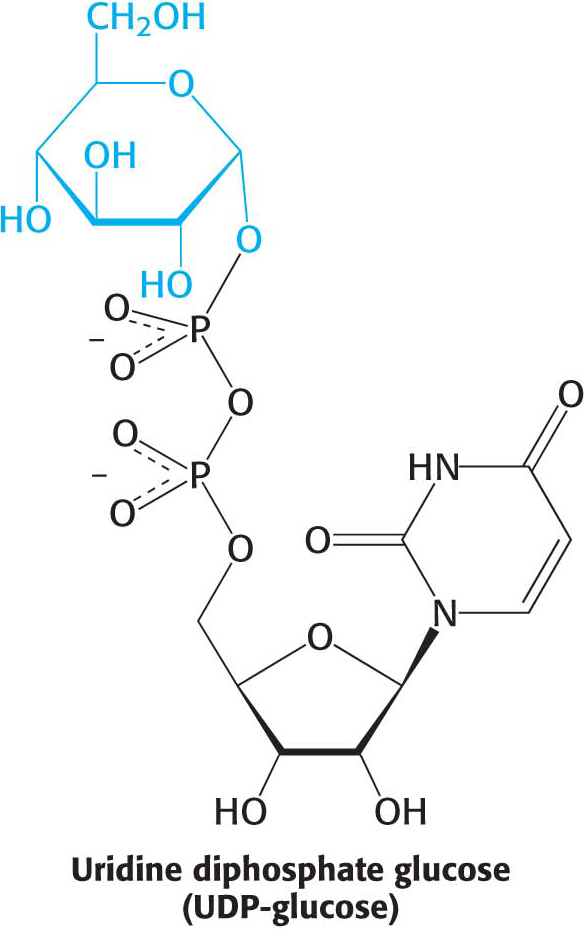
UDP-
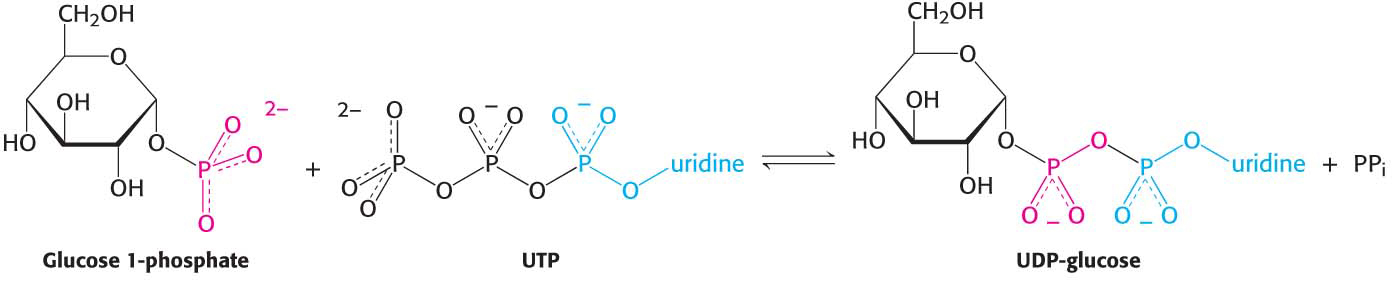
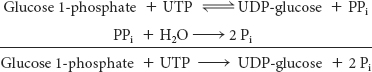
This reaction is readily reversible. However, pyrophosphate is rapidly hydrolyzed in vivo to orthophosphate by an inorganic pyrophosphatase. The essentially irreversible hydrolysis of pyrophosphate drives the synthesis of UDP-
The synthesis of UDP-
Glycogen Synthase Catalyzes the Transfer of Glucose from UDP-Glucose to a Growing Chain
New glucosyl units are added to the nonreducing terminal residues of glycogen. The activated glucosyl unit of UDP-
Glycogen synthase can add glucosyl residues only to a polysaccharide chain already containing more than four residues. Thus, glycogen synthesis requires a primer. This priming function is carried out by glycogenin, an enzyme composed of two identical 37-
A Branching Enzyme Forms Alpha-1,6 Linkages
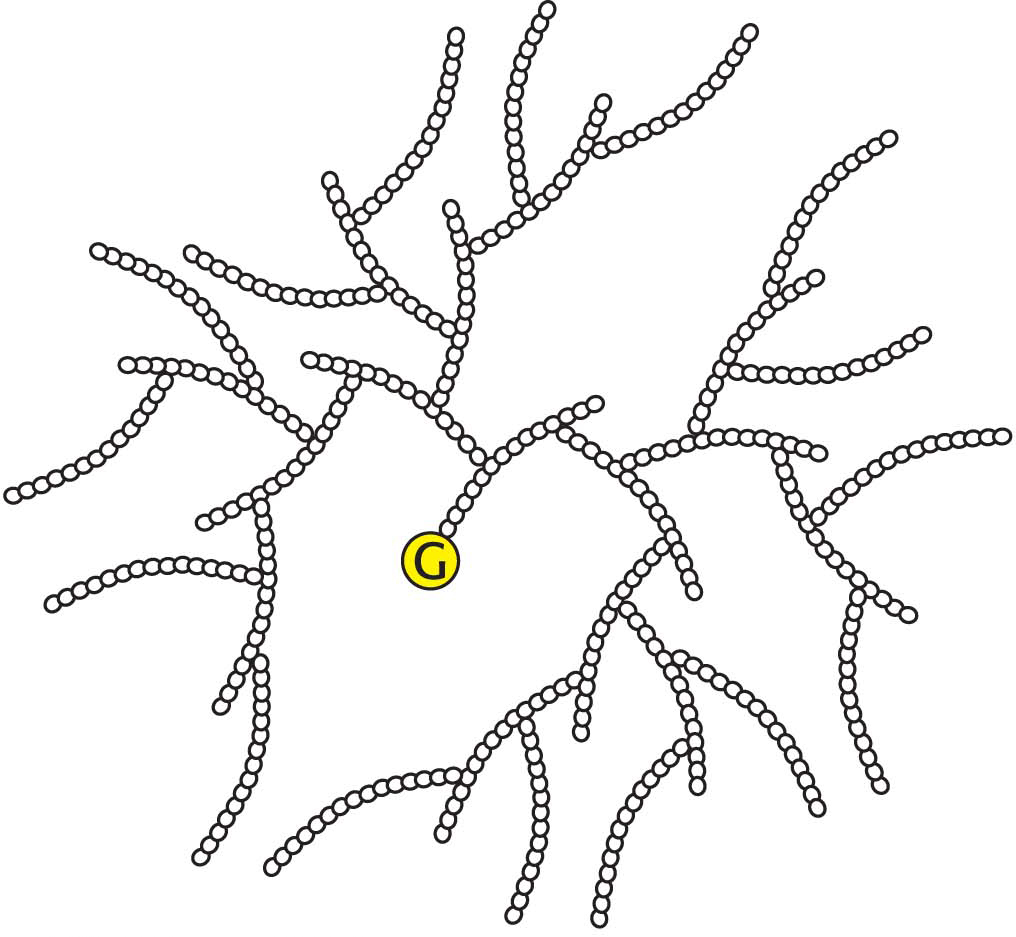
Glycogen synthase catalyzes only the synthesis of α-1,4 linkages. Another enzyme is required to form the α-1,6 linkages that make glycogen a branched polymer. Branching takes place after a number of glucosyl residues are joined in α-1,4 linkages by glycogen synthase. A branch is created by the breaking of an α-1,4 link and the formation of an α-1,6 link. A block of residues, typically 7 in number, is transferred to a more interior site. The branching enzyme that catalyzes this reaction is quite exacting (Figure 25.2). The block of 7 or so residues must include the nonreducing terminus and come from a chain at least 11 residues long. In addition, the new branch point must be at least 4 residues away from a preexisting one.
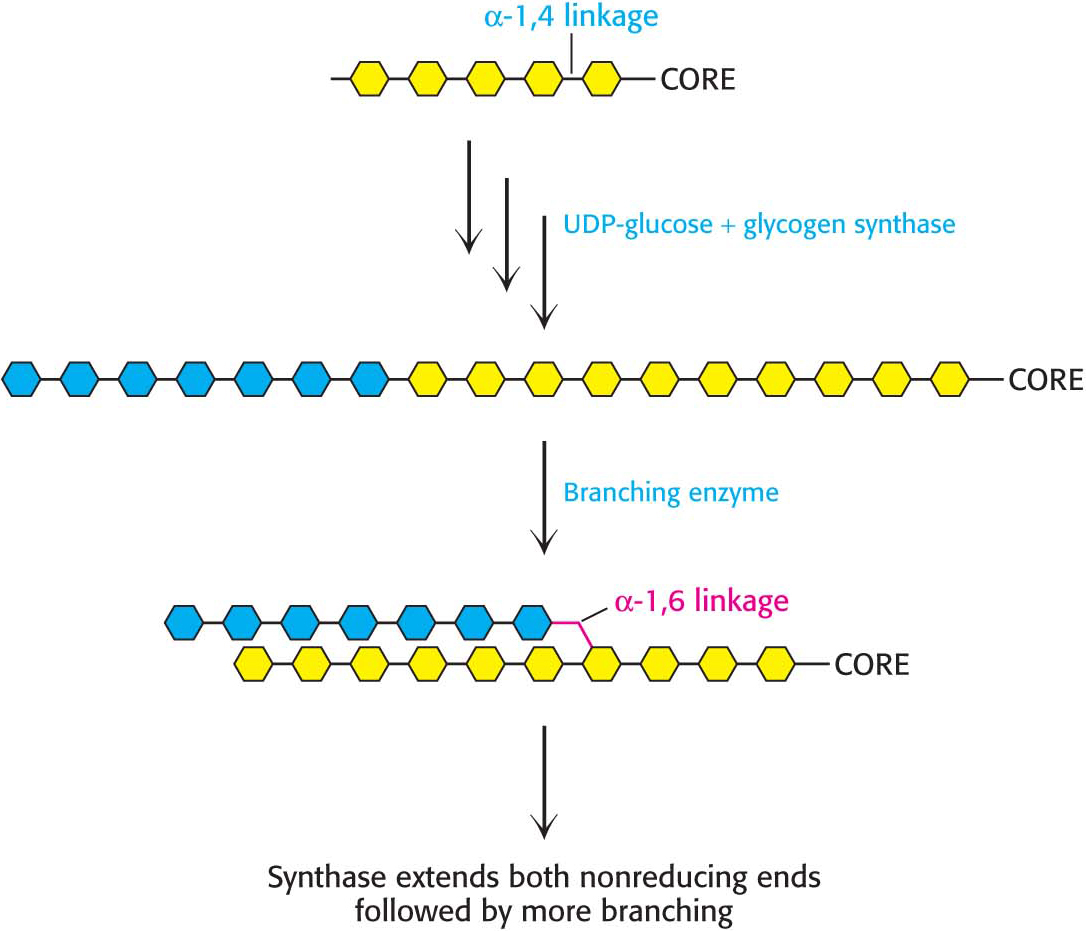
Branching is important because it increases the solubility of glycogen. Furthermore, branching creates a large number of terminal residues, the sites of action of glycogen phosphorylase and synthase (Figure 25.1). Thus, branching increases the rate of glycogen synthesis and degradation.
Glycogen Synthase Is the Key Regulatory Enzyme in Glycogen Synthesis
Glycogen synthase, like phosphorylase, exists in two forms: an active nonphosphorylated a form and a usually inactive phosphorylated b form. Again, like the phosphorylase, the interconversion of the two forms is regulated by covalent modification. However, the key means of regulating glycogen synthase is by allosteric regulation of the phosphorylated form of the enzyme, glycogen synthase b. Glucose 6-
QUICK QUIZ 1
Why is the fact that phosphorylation has opposite effects on glycogen synthesis and breakdown advantageous?
It prevents synthesis and breakdown from taking place simultaneously, which would lead to a useless expenditure of energy. See problem 9 in the Problems section.
The covalent modification of glycogen synthase appears to play more of a fine-
Glycogen Is an Efficient Storage Form of Glucose
What is the cost of converting dietary glucose into glycogen and then into glucose 6-
DID YOU KNOW?
Studies have shown that, when muscle glycogen is depleted, the power output of the muscle falls to approximately 50% of maximum. Power output decreases despite the fact that ample supplies of fat are available, suggesting that fats can supply only about 50% of maximal aerobic effort. If carbohydrate-
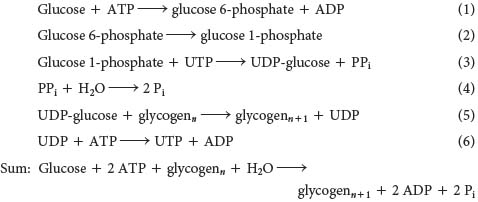
Thus, 2 molecules of ATP are hydrolyzed to incorporate dietary glucose into glycogen. The energy yield from the breakdown of glycogen formed from dietary glucose is highly efficient. About 90% of the residues are cleaved by phosphorolysis to glucose 1-