PROBLEMS
Question 25.1
1. Yin and Yang. Match the terms on the left with the descriptions on the right. ✓ 3
UDP- UDP- Glycogen synthase Glycogenin Branching enzyme Glucose 6- Glycogen synthase kinase Protein phosphatase 1 Insulin Glycogen phosphorylase a | Glucose sensor in the liver. Synthesizes α-1,4 linkages between glucose molecules. Catalyzes the formation of glycogen synthase b. Synthesizes the primer for glycogen synthesis. Catalyzes the formation of glycogen synthase a. Activated substrate for glycogen synthesis. Synthesizes α-1,6 linkages between glucose molecules. Potent activator of glycogen synthase b. Leads to the inactivation of glycogen synthase kinase. Glucose 1- |
Question 25.2
2. Team effort. What enzymes are required for the synthesis of a glycogen particle starting from glucose 6-
Question 25.3
3. ATP is behind everything! UDP-
Question 25.4
4. Force it forward. The following reaction accounts for the synthesis of UDP-
Question 25.5
5. If you insist. Why does activation of the phosphorylated b form of glycogen synthase by high concentrations of glucose 6-
Question 25.6
6. Initiate and extend. Describe the separate roles of glycogenin and glycogen synthase in glycogen synthesis. ✓ 4
Question 25.7
7. An ATP saved is an ATP earned. The complete oxidation of glucose 6-
Question 25.8
8. Dual roles. Phosphoglucomutase is crucial for glycogen breakdown as well as for glycogen synthesis. Explain the role of this enzyme in each of the two processes.
Question 25.9
9. Working at cross-
Question 25.10
10. Achieving immortality. Glycogen synthase requires a primer. The primer was once thought to be provided when the existing glycogen granules are divided between the daughter cells produced by cell division. In other words, parts of the original glycogen molecule were simply passed from generation to generation. Would this strategy have been successful in passing glycogen stores from generation to generation? How are new glycogen molecules now known to be synthesized? ✓ 4
Question 25.11
11. Synthesis signal. How does insulin stimulate glycogen synthesis? ✓ 4
Question 25.12
12. Excessive storage. Suggest an explanation for the fact that the amount of glycogen in type I glycogen-
Chapter Integration Problems
Question 25.13
13. Metabolic mutants. Predict the major consequence of each of the following mutations. ✓ 5
(a) Loss of the AMP-
(b) Mutation of Ser 14 to Ala 14 in liver phosphorylase.
(c) Overexpression of phosphorylase kinase in the liver.
(d) Loss of the gene that encodes the inhibitor of protein phosphatase 1.
(e) Loss of the gene that encodes the glycogen-
(f) Loss of the gene that encodes glycogenin.
Question 25.14
14. More metabolic mutants. Briefly predict the major consequences of each of the following mutations affecting glycogen utilization. ✓ 5
(a) Loss of GTPase activity of the G-
(b) Loss of phosphodiesterase activity.
Question 25.15
15. Same symptoms, different cause. Von Gierke disease is frequently the result of a defect in glucose 6-
Question 25.16
16. Again, von Gierke. People suffering from von Gierke disease release a small amount of glucose into the blood after the injection of glucagon. How is this result possible?
Question 25.17
17. I know I’ve seen that face before. UDP-
Question 25.18
18. Carbohydrate conversion. Write a balanced equation for the formation of glycogen from galactose. ✓ 3
Chapter Integration, Data Interpretation, and Challenge Problems
Question 25.19
19. Removing all traces. In human liver extracts, the catalytic activity of glycogenin was detectable only after treatment with α-amylase, an enzyme that hydrolyzes α-1,4-
Question 25.20
20. Telltale products. A sample of glycogen from a patient with liver disease is incubated with orthophosphate, phosphorylase, the transferase, and the debranching enzyme (α-1,6-
Question 25.21
21. Glycogen isolation 1. The liver is a major storage site for glycogen. Purified from two samples of human liver, glycogen was either treated or not treated with α-amylase and subsequently analyzed by SDS-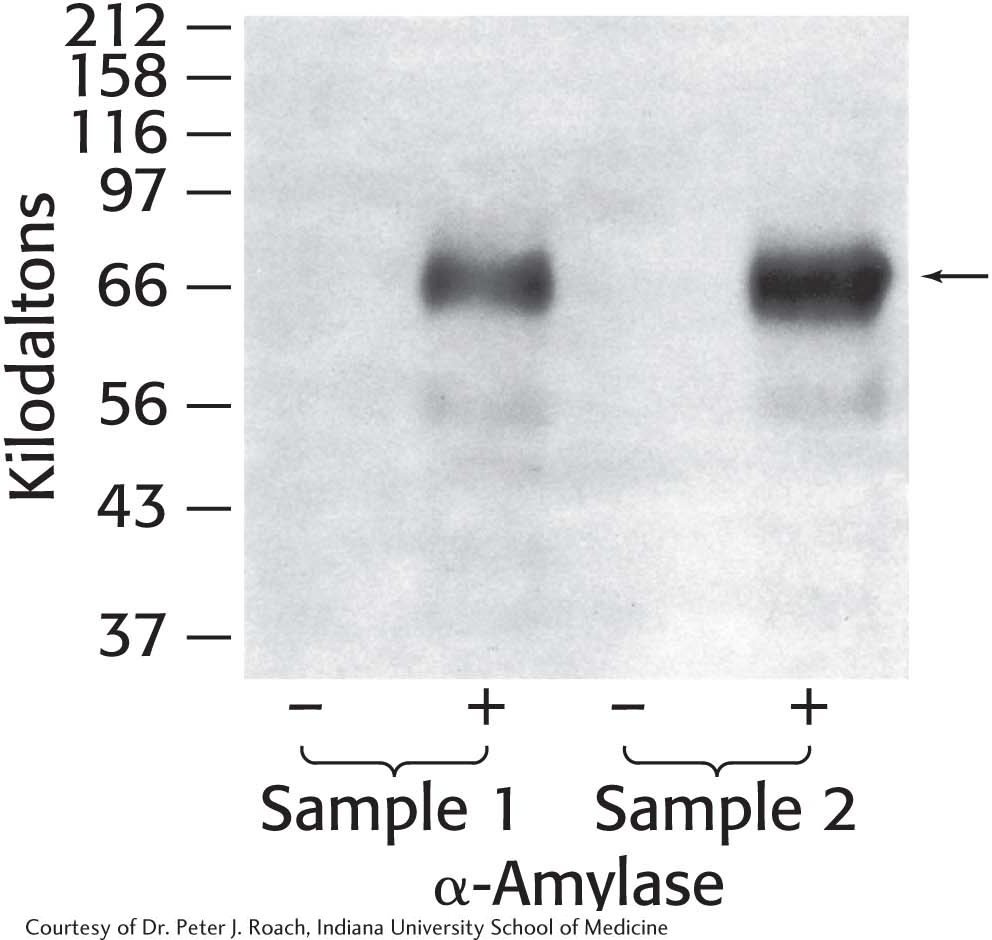
(a) Why are no proteins visible in the lanes without amylase treatment?
(b) What is the effect of treating the samples with α-amylase? Explain the results.
(c) List other proteins that you might expect to be associated with glycogen. Why are other proteins not visible?
Question 25.22
22. Glycogen isolation 2. The gene for glycogenin was transfected into a cell line that normally stores only small amounts of glycogen. The cells were then manipulated according to the following protocol, and glycogen was isolated and analyzed by SDS-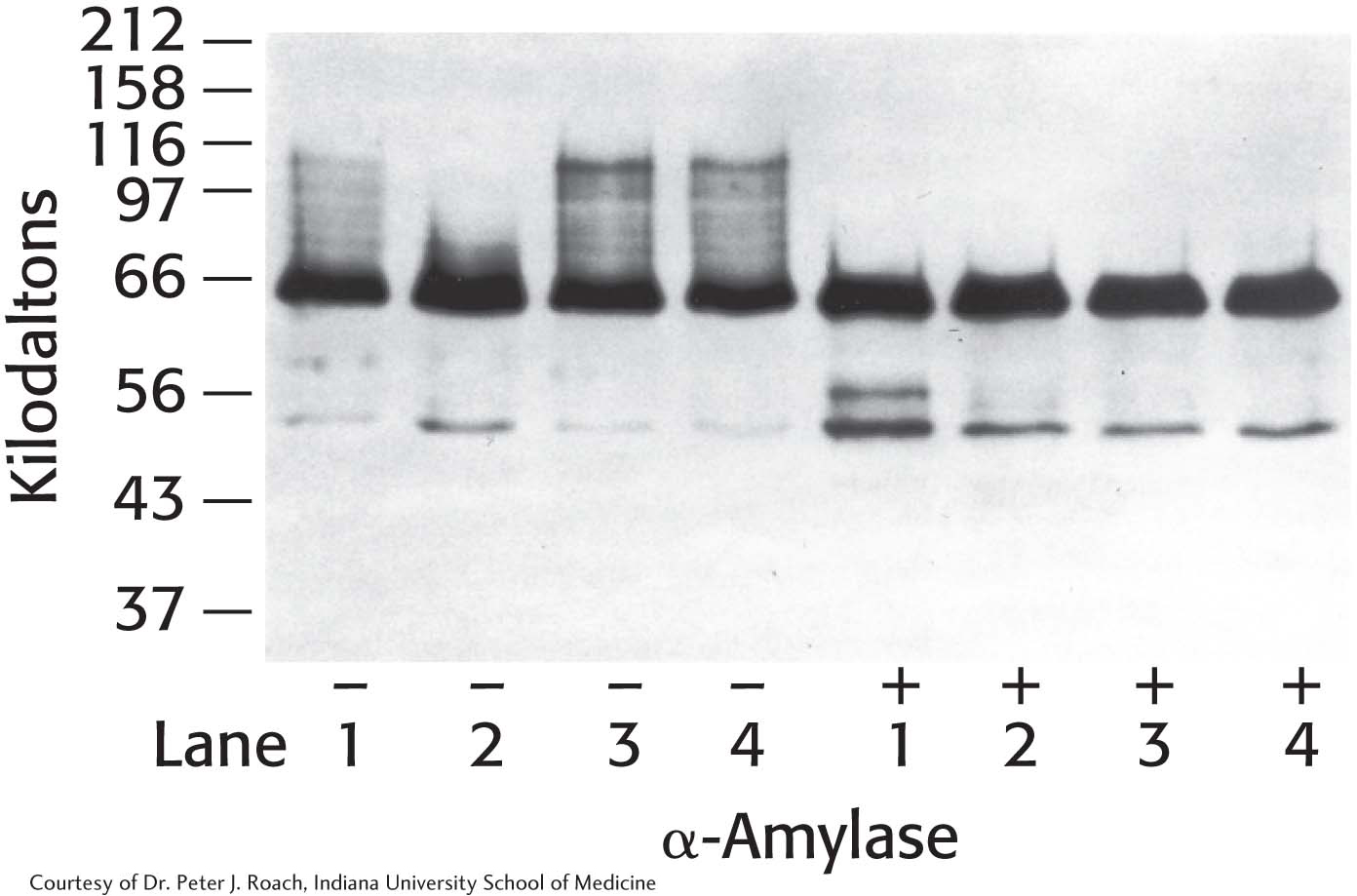
The protocol: Cells cultured in growth medium and 25 mM glucose (lane 1) were switched to medium containing no glucose for 24 hours (lane 2). Glucose-
(a) Why did the western analysis produce a “smear”—that is, the high-
(b) What is the significance of the decrease in high-
(c) What is the significance of the difference between lanes 2(-) and 3(-)?
(d) Suggest a plausible reason why there is essentially no difference between lanes 3(-) and 4(-)?
(e) Why are the bands at 66 kDa the same in the lanes treated with α-amylase, despite the fact that the cells were treated differently?
Selected Readings for this chapter can be found online at www.whfreeman.com/