
27.1 Fatty Acids Are Processed in Three Stages
✓ 1 Identify the repeated steps of fatty acid degradation.
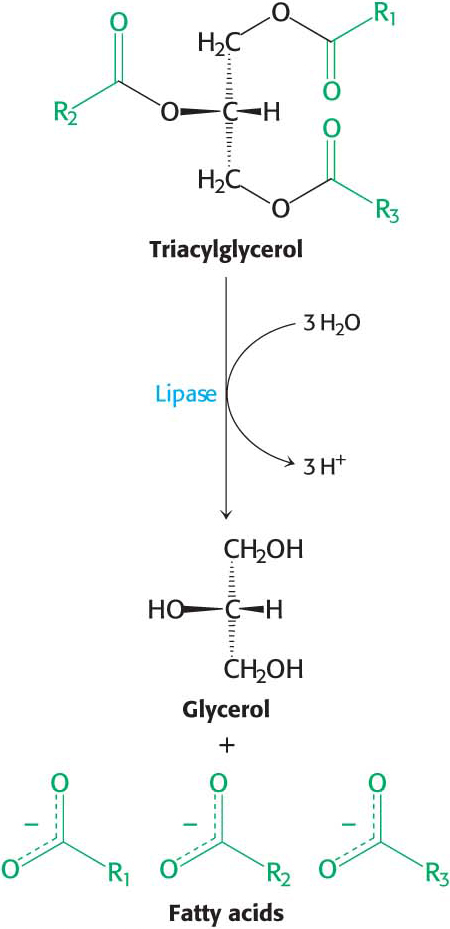
In Chapter 14, we examined how dietary triacylglycerols are digested, absorbed, transported, and stored. Now, we will examine how the stored triacylglycerols are made biochemically accessible. Peripheral tissues, such as muscle, gain access to the lipid energy reserves stored in adipose tissue through three stages of processing. First, the lipids must be mobilized. In this process of lipolysis, triacylglycerols are degraded to fatty acids and glycerol, which are released from the adipose tissue and transported to the energy-
 CLINICAL INSIGHT
CLINICAL INSIGHTTriacylglycerols Are Hydrolyzed by Hormone–Stimulated Lipases
Consider someone who has just awakened from a night’s sleep and begins a bout of exercise. After the night’s fast, glycogen stores are low, but lipids are readily available. How are these lipid stores mobilized to provide fuel for muscles and other tissues?
Triacylglycerols are stored inside a fat cell (adipocyte) as a lipid droplet, an intracellular compartment surrounded by a single layer of phospholipids and the numerous proteins required for fatty acid metabolism (Figure 11.3). Originally believed to be inert-
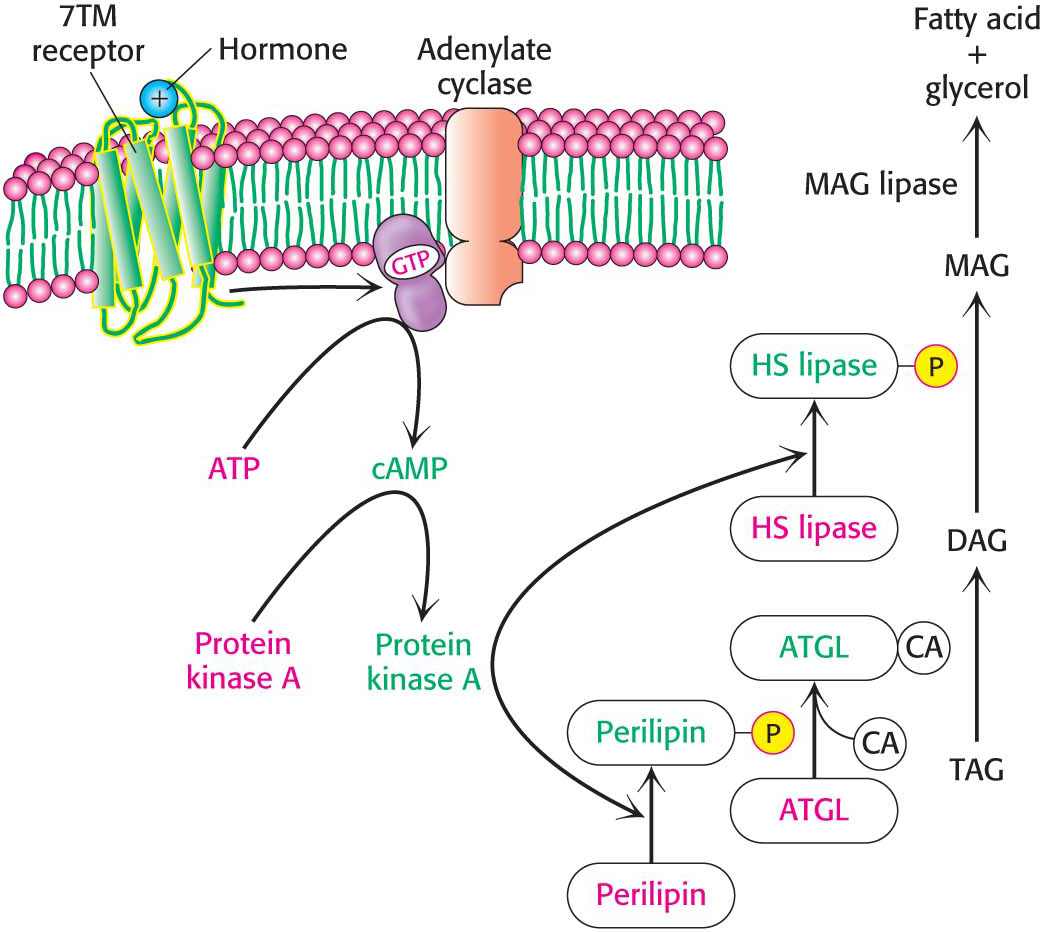
If the coactivator required by ATGL is missing or defective, a rare condition called Chanarin-
Free Fatty Acids and Glycerol Are Released Into the Blood
Fatty acids are not soluble in aqueous solutions. In order to reach tissues that require fatty acids, the released fatty acids bind to the blood protein albumin, which delivers them to tissues in need of fuel.
Glycerol formed by lipolysis is absorbed by the liver and phosphorylated. It is then oxidized to dihydroxyacetone phosphate, which is isomerized to glyceraldehyde 3-
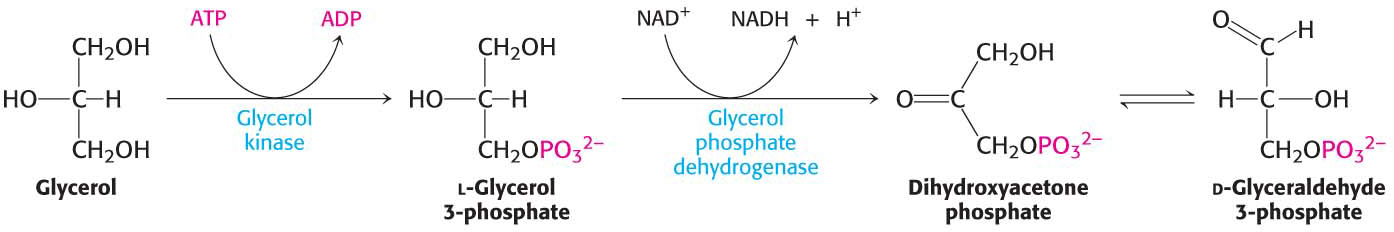
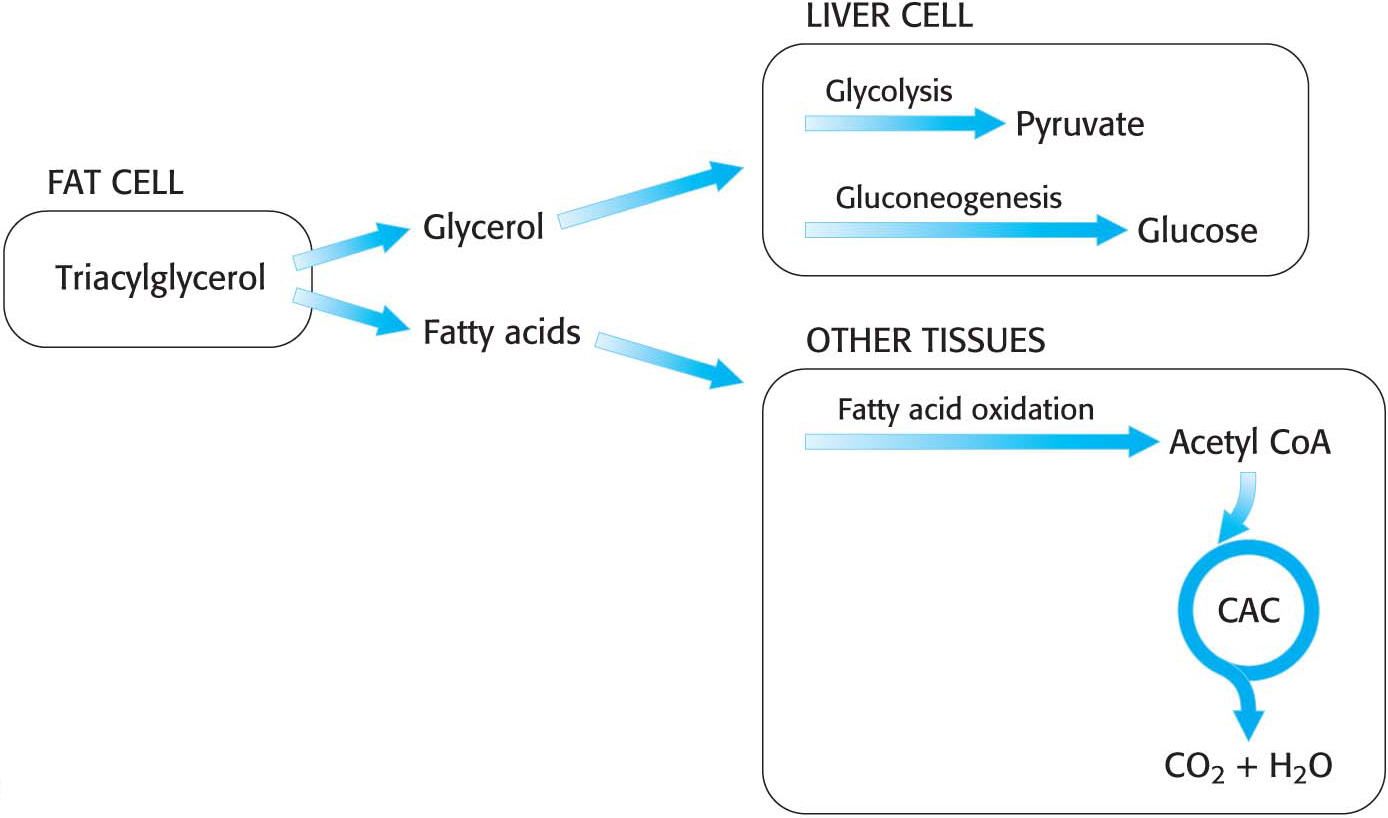
Hence, glycerol can be converted into pyruvate or glucose in the liver, which contains the appropriate enzymes (Figure 27.3). The reverse reaction can take place just as readily. Thus, glycerol and glycolytic intermediates are interconvertible.
Fatty Acids Are Linked to Coenzyme A Before They Are Oxidized
Fatty acids separate from the albumin in the bloodstream and diffuse across the cell membrane with the assistance of transport proteins. In the cell, fatty acids are shuttled about in association with fatty-
Fatty acid oxidation takes place in mitochondria, so how do these fuels gain access to the site of degradation? First, fatty acids must be activated by reacting with coenzyme A to form acyl CoA. This activation reaction takes place on the outer mitochondrial membrane, where it is catalyzed by acyl CoA synthetase.
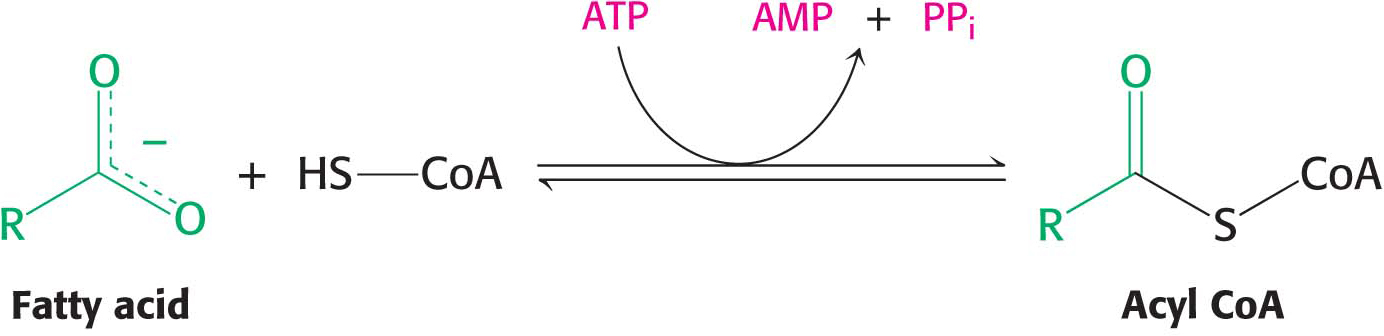
The activation takes place in two steps:
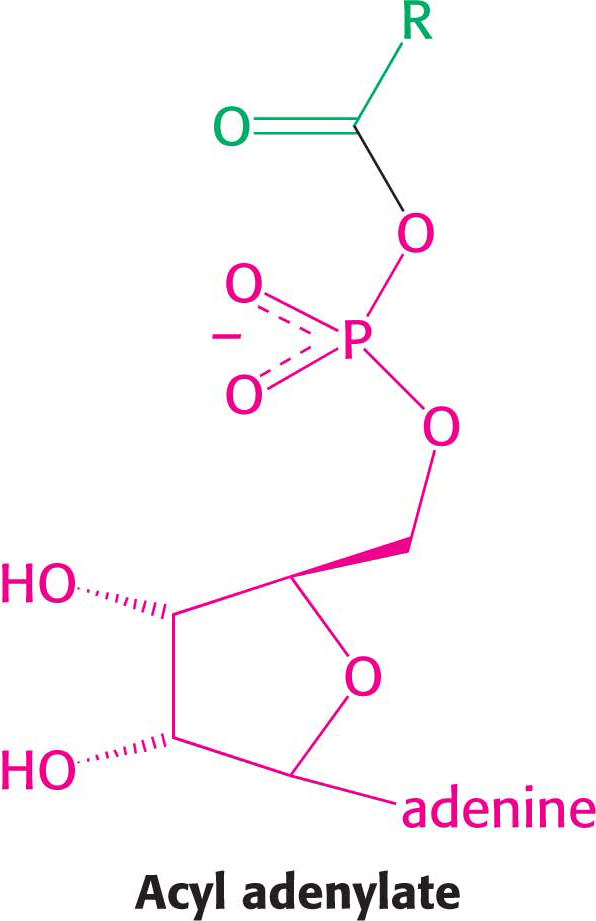
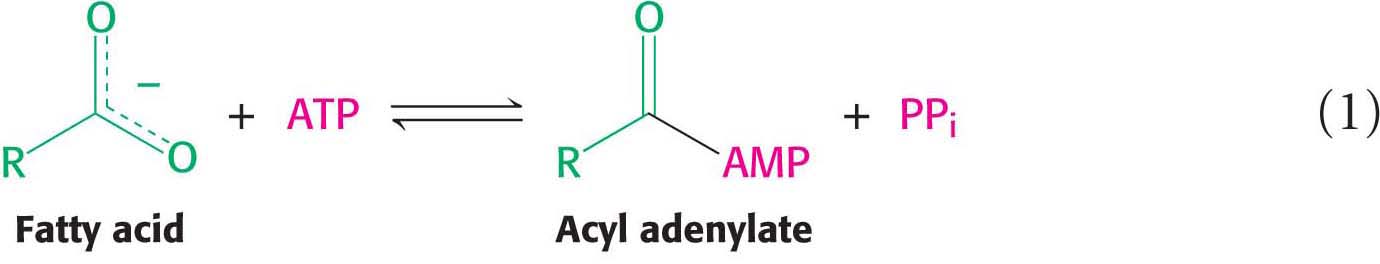
The fatty acid reacts with ATP to form an acyl adenylate, and the other two phosphoryl groups of the ATP substrate are released as pyrophosphate:
The sulfhydryl group of CoA then attacks the acyl adenylate to form acyl CoA and AMP:
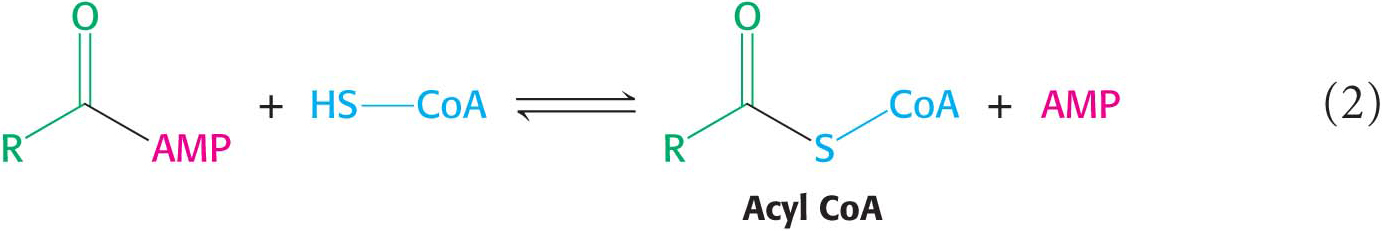
These partial reactions are freely reversible. In fact, the equilibrium constant for the sum of these reactions is close to 1, meaning that the energy levels of the reactants and products are about equal. The reaction is driven forward by the hydrolysis of pyrophosphate by pyrophosphatase:
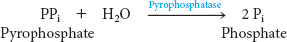
We see here another example of a recurring theme in biochemistry: many biosynthetic reactions are made irreversible by the hydrolysis of inorganic pyrophosphate. Thus, the complete reaction for fatty acid activation is
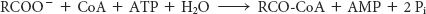
Activation is not the only step necessary to move fatty acids into the mitochondrial matrix. Activated fatty acids can cross the outer mitochondrial membrane through the voltage-
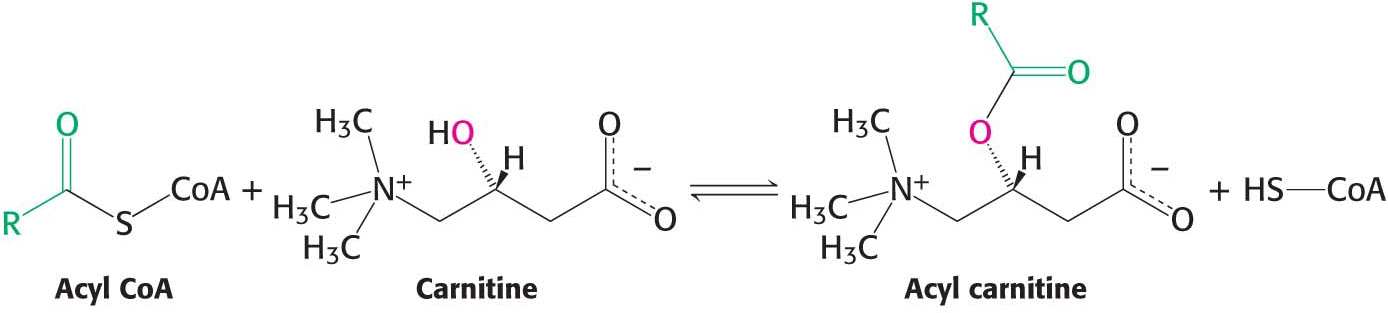
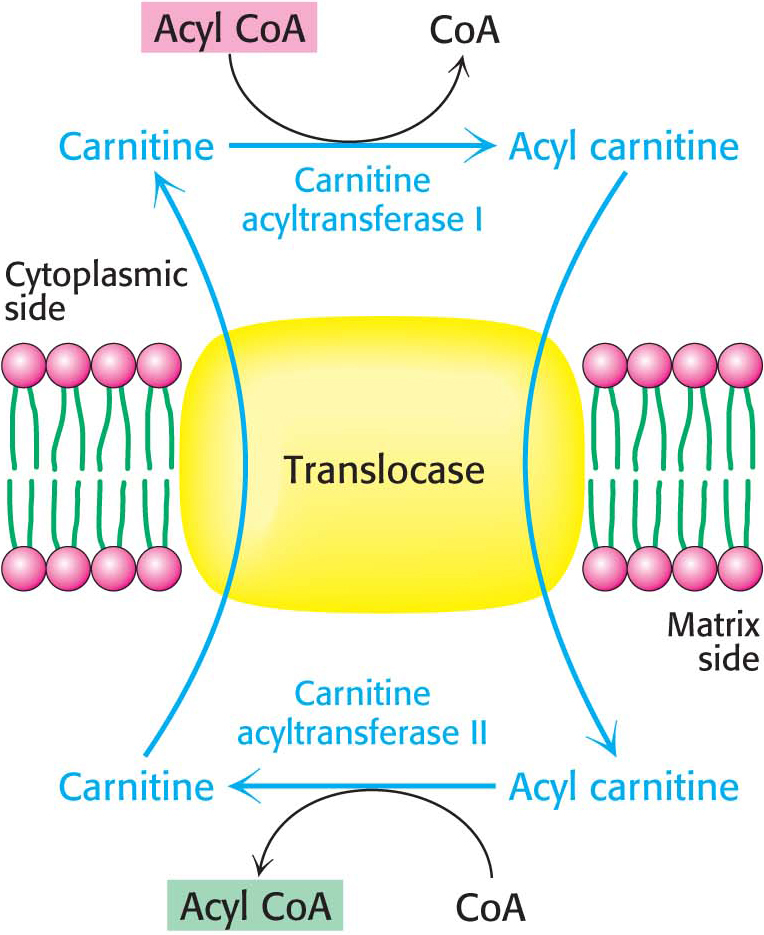
Acyl carnitine is then shuttled across the inner mitochondrial membrane by a translocase (Figure 27.4). The acyl group is transferred back to CoA by carnitine acyltransferase II (carnitine palmitoyl transferase II) on the matrix side of the membrane. Finally, the translocase returns carnitine to the cytoplasmic side in exchange for an incoming acyl carnitine, allowing the process to continue.
 CLINICAL INSIGHT
CLINICAL INSIGHTPathological Conditions Result if Fatty Acids Cannot Enter the Mitochondria
A number of diseases have been traced to a deficiency of carnitine, carnitine transferase, or translocase. The symptoms of carnitine deficiency range from mild muscle cramping to severe weakness and even death. Inability to synthesize carnitine may be a contributing factor to the development of autism in males. In general, muscle, kidney, and heart are the tissues primarily impaired. Muscle weakness during prolonged exercise is a symptom of a deficiency of carnitine acyltransferases because muscle relies on fatty acids as a long-
Acetyl CoA, NADH, and FADH2 Are Generated by Fatty Acid Oxidation
After the activated fatty acid is in mitochondria, it is ready for metabolism. The goal of fatty acid degradation is to oxidize the fatty acid—
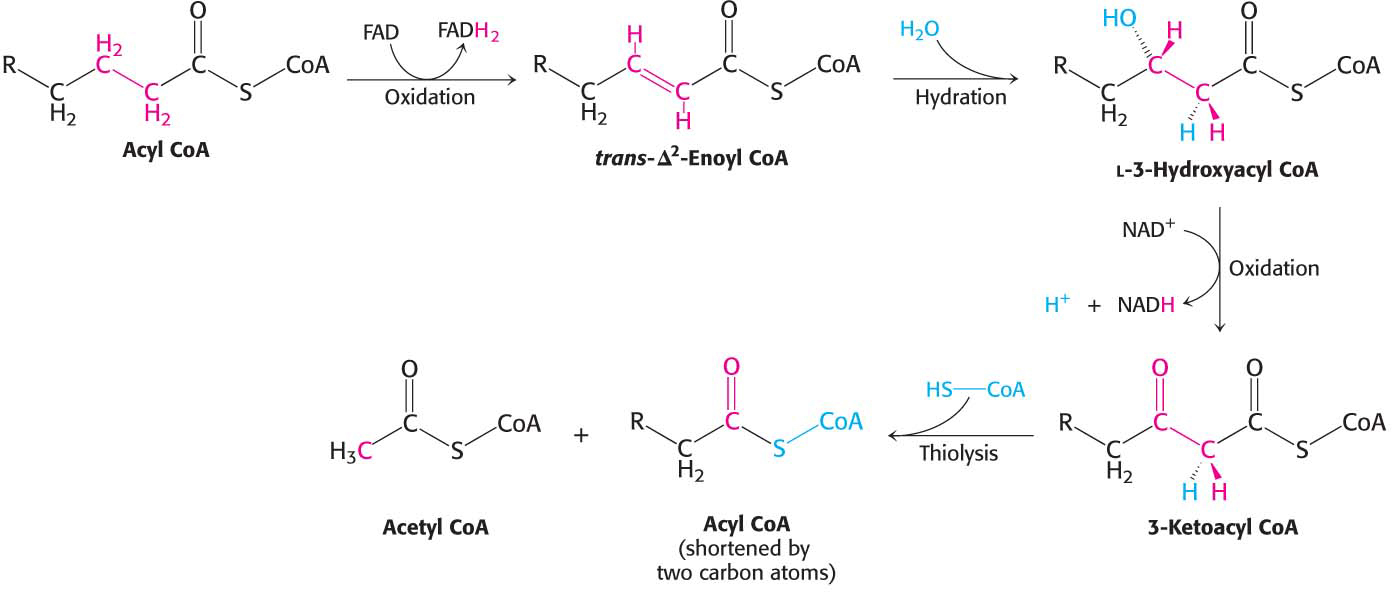
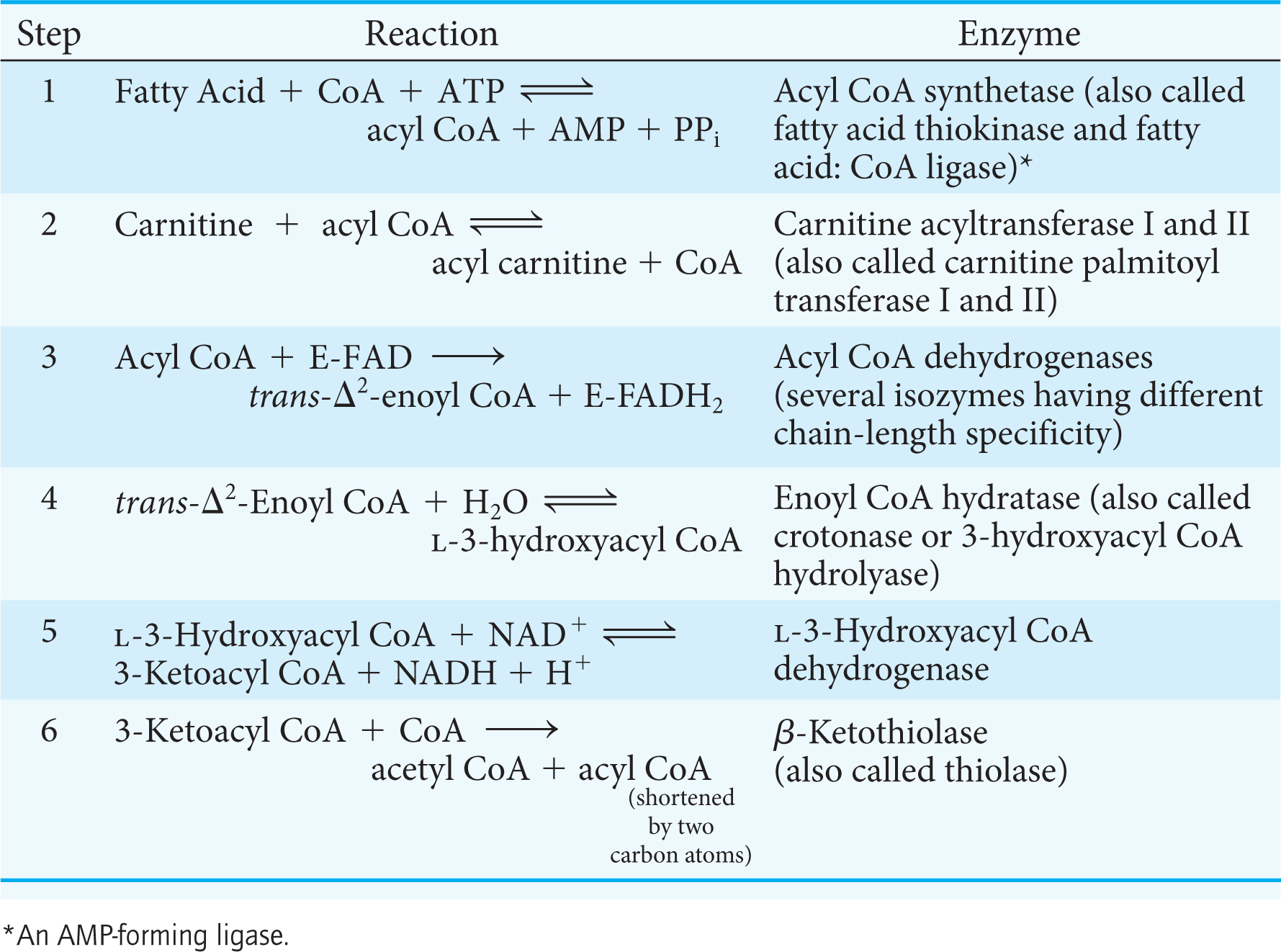
The first reaction in each round of degradation is the oxidation of acyl CoA by an acyl CoA dehydrogenase to give an enoyl CoA with a trans double bond between C-
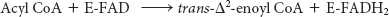
DID YOU KNOW?
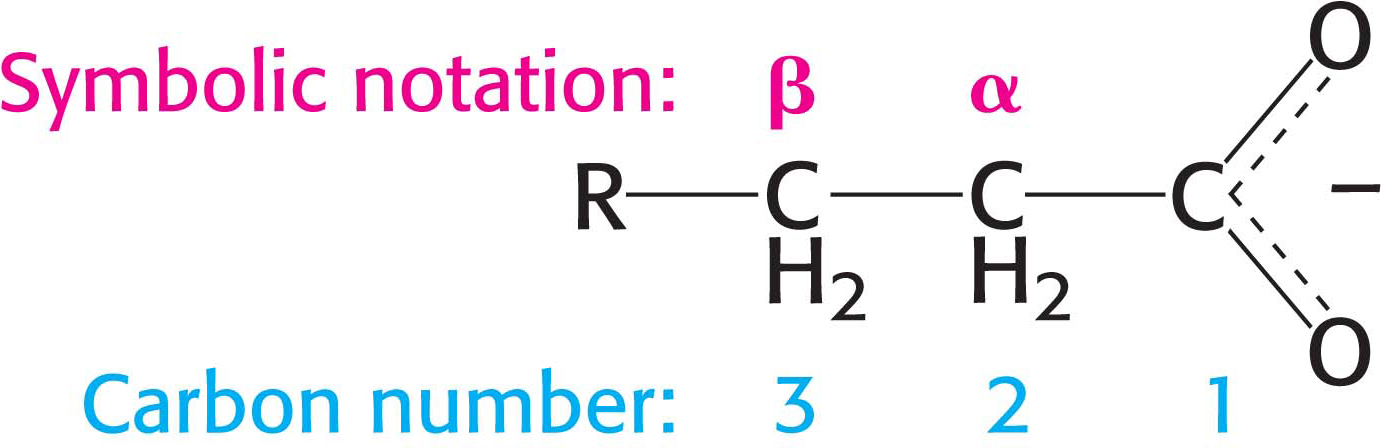
The symbol Δn (capital delta, superscript number) is used to denote the position of the first carbon atom participating in a double bond. Thus, Δ2 designates a double bond between carbon 2 and carbon 3.
As in the dehydrogenation of succinate in the citric acid cycle, FAD rather than NAD+ is the electron acceptor because the ΔG for this reaction is insufficient to drive the reduction of NAD+. Electrons picked up by FAD are transferred to the electron-
The next step is the hydration of the double bond between C-
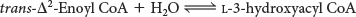
The hydration of enoyl CoA is stereospecific. Only the l isomer of 3-
The hydration of enoyl CoA is a prelude to the second oxidation reaction, which converts the hydroxyl group at C-

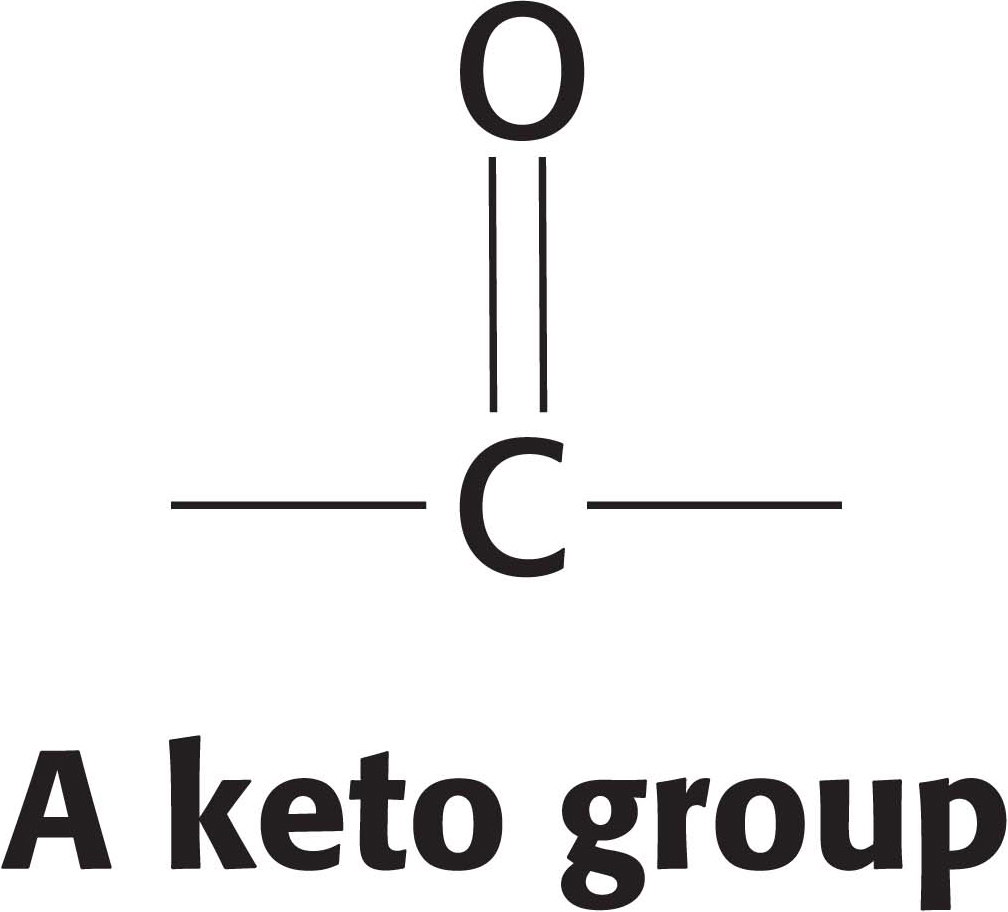
The preceding reactions have oxidized the methylene group (—CH2—) at C-
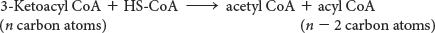
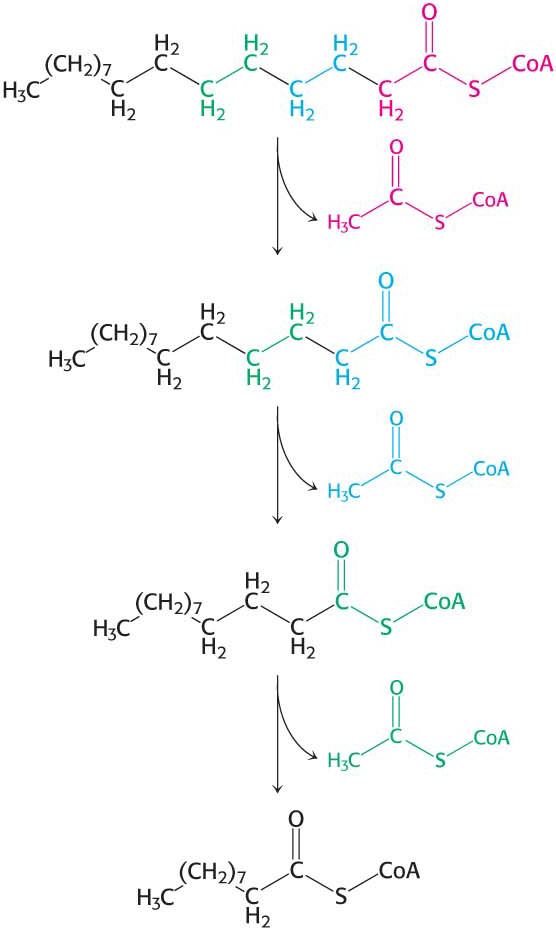
Table 27.1 summarizes the reactions in fatty acid degradation. The shortened acyl CoA then undergoes another cycle of oxidation, starting with the reaction catalyzed by acyl CoA dehydrogenase (Figure 27.6).
Recent evidence suggests that the enzymes of fatty acid oxidation are associated with one another to form a super complex. Moreover, this super complex is in turn associated with the inner mitochondrial membrane, the site of the electron-
The Complete Oxidation of Palmitate Yields 106 Molecules of ATP
We can now calculate the energy yield derived from the oxidation of a fatty acid. In each reaction cycle, an acyl CoA is shortened by two carbon atoms, and one molecule each of FADH2, NADH, and acetyl CoA is formed:
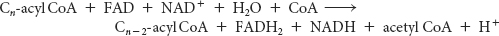
The degradation of palmitoyl CoA (C16-acyl CoA) requires seven reaction cycles. In the seventh cycle, the C4-ketoacyl CoA is cleaved by the thiol group of coenzyme A to two molecules of acetyl CoA. Hence, the stoichiometry of the oxidation of palmitoyl CoA is
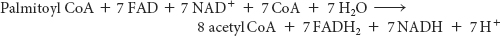
QUICK QUIZ 1
Describe the repetitive steps of β oxidation. Why is the process called β oxidation?
The steps are (1) oxidation by FAD; (2) hydration; (3) oxidation by NAD+; (4) thiolysis to yield acetyl CoA. In symbolic notation, the β-carbon atom is oxidized.
Approximately 2.5 molecules of ATP are generated when the respiratory chain oxidizes each of these NADH molecules, whereas 1.5 molecules of ATP are formed for each FADH2 because their electrons enter the chain at the level of ubiquinol. Recall that the oxidation of acetyl CoA by the citric acid cycle yields 10 molecules of ATP. Hence, the number of ATP molecules formed in the oxidation of palmitoyl CoA is 10.5 from the 7 molecules of FADH2, 17.5 from the 7 molecules of NADH, and 80 from the 8 molecules of acetyl CoA, which gives a total of 108. The equivalent of 2 molecules of ATP is consumed in the activation of palmitate, in which ATP is split into AMP and two molecules of orthophosphate. Thus, the complete oxidation of a molecule of palmitate yields 106 molecules of ATP.