27.2 The Degradation of Unsaturated and Odd-Chain Fatty Acids Requires Additional Steps
The β-oxidation pathway accomplishes the complete degradation of saturated fatty acids having an even number of carbon atoms. Most fatty acids have such structures because of their mode of synthesis (Chapter 28). However, not all fatty acids are so simple. The oxidation of fatty acids containing double bonds requires additional steps. Likewise, fatty acids containing an odd number of carbon atoms require additional enzyme reactions to yield a metabolically useful molecule.
An Isomerase and a Reductase Are Required for the Oxidation of Unsaturated Fatty Acids
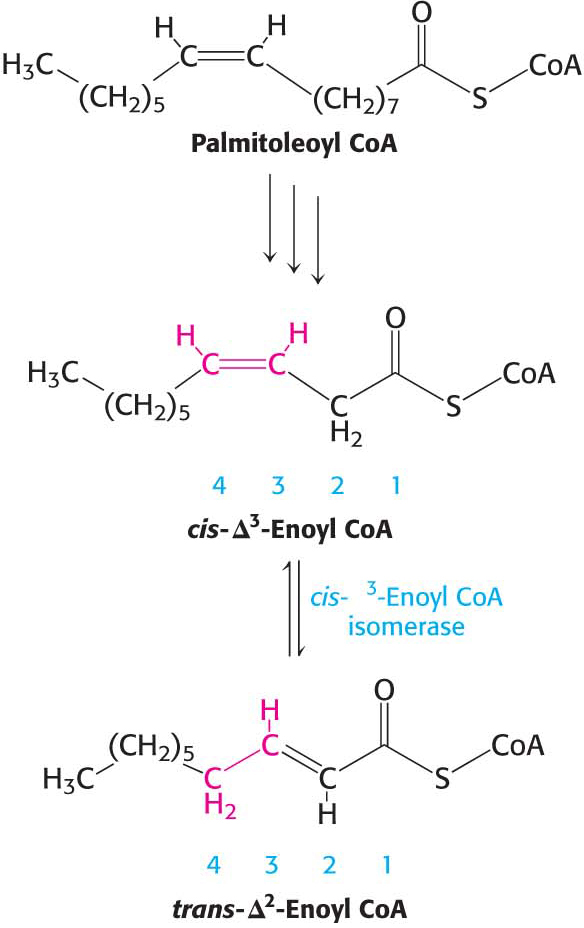
Many unsaturated fatty acids are available in our diet. Indeed, we are encouraged to eat foods that are rich in certain types of polyunsaturated fatty acids, such as the ω-3 fatty acid linolenic acid, which is prominent in safflower and corn oils. Polyunsaturated fatty acids are important for a number of reasons, not the least of which is that they offer some protection from heart attacks. How are excess amounts of these fatty acids oxidized?
NUTRITION FACTS

Consider the oxidation of palmitoleate. This C16 unsaturated fatty acid, which has one double bond between C-
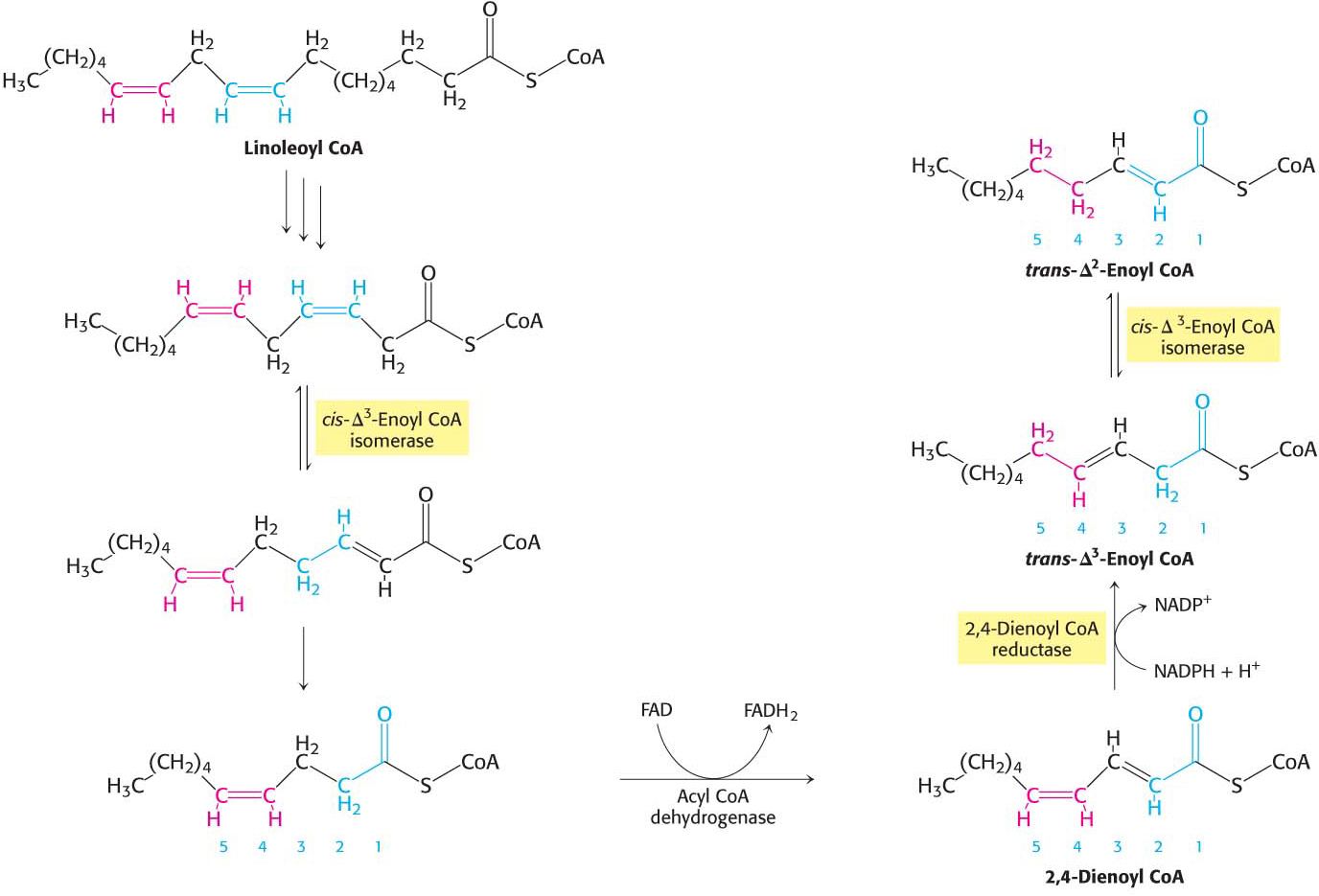
Human beings require polyunsaturated fatty acids, which have multiple double bonds, as important precursors of signal molecules, but excess polyunsaturated fatty acids are degraded by β oxidation. However, when these fats are subjected to β oxidation, molecules result that cannot themselves be degraded by β oxidation. To prevent a wasteful buildup of these molecules, the initial degradation products of the polyunsaturated fatty acids must first be modified. Consider linoleate, a C18 polyunsaturated fatty acid with cis-Δ9 and cis-Δ12 double bonds (Figure 27.8). The cis-Δ3 double bond formed after three rounds of β oxidation is converted into a trans-Δ2 double bond by the aforementioned isomerase. The acyl CoA produced by another round of β oxidation contains a cis-Δ4 double bond. The dehydrogenation of this species by acyl CoA dehydrogenase yields a 2,4-
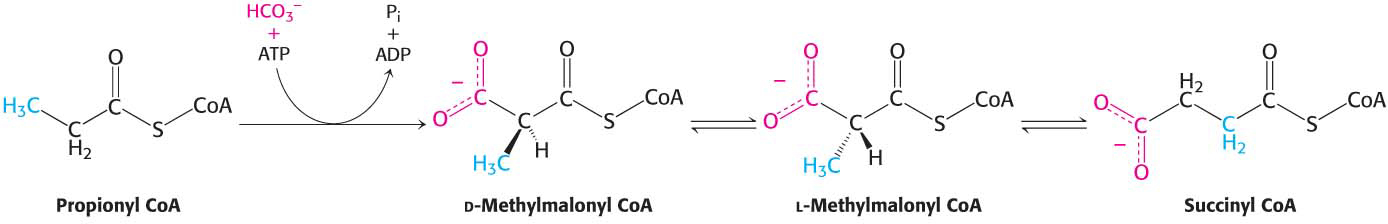
Odd-Chain Fatty Acids Yield Propionyl CoA in the Final Thiolysis Step
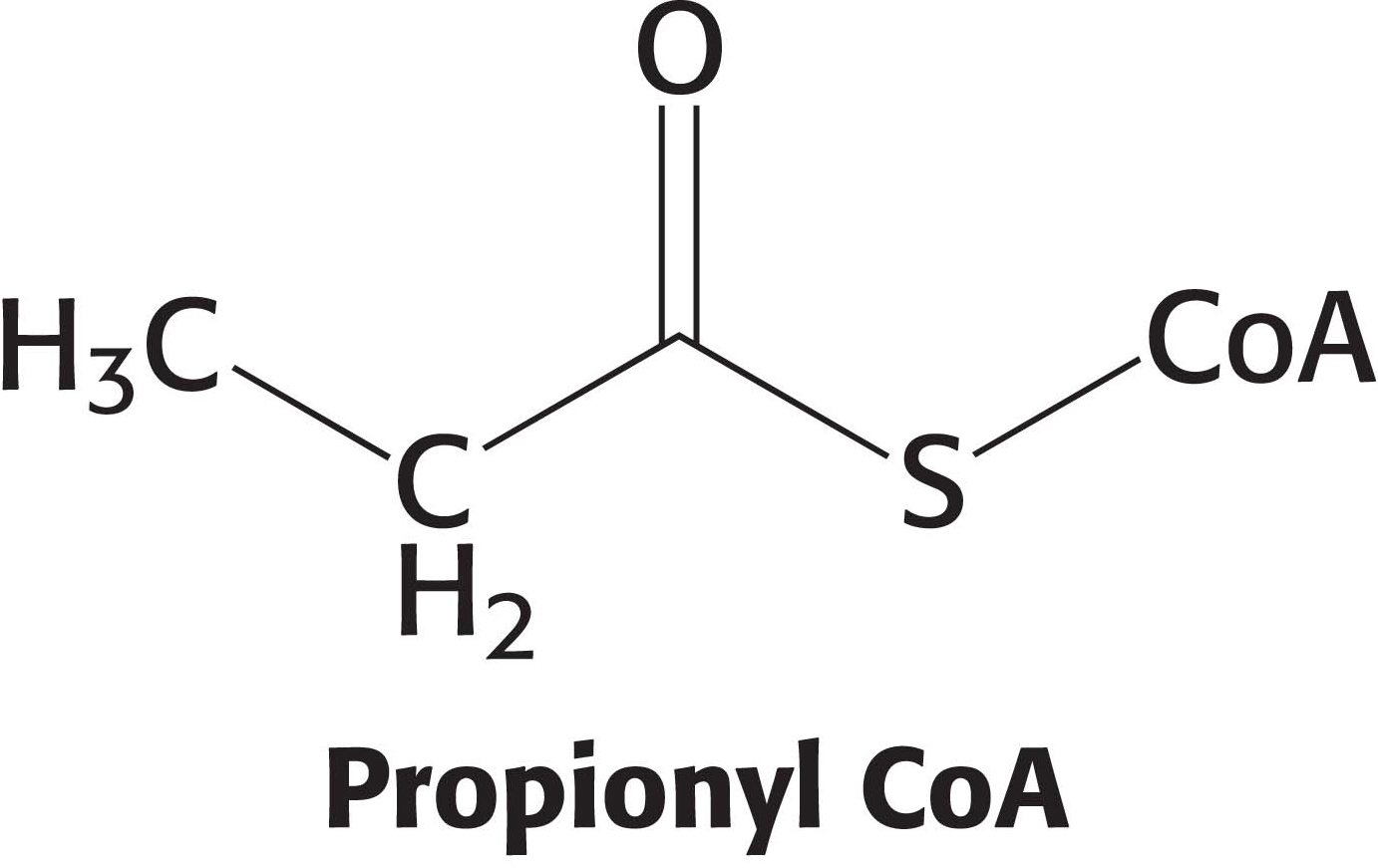
Fatty acids having an odd number of carbon atoms are a minor class found in small amounts in vegetables. They are oxidized in the same way as fatty acids having an even number of carbon atoms, except that propionyl CoA and acetyl CoA, rather than two molecules of acetyl CoA, are produced in the final round of degradation. The activated three-
The pathway from propionyl CoA to succinyl CoA is especially interesting because it entails a rearrangement that requires vitamin B12 (also known as cobalamin). Propionyl CoA is carboxylated by propionyl CoA carboxylase (a biotin enzyme) at the expense of the hydrolysis of a molecule of ATP to yield the d isomer of methylmalonyl CoA (Figure 27.9). The d isomer of methylmalonyl CoA is converted into the l isomer, the substrate for a mutase that converts it into succinyl CoA by an intramolecular rearrangement. The —CO—