
27.4 Metabolism in Context: Fatty Acid Metabolism Is a Source of Insight into Various Physiological States
Fatty acids are our most prominent fuel. Not surprisingly then, the metabolism of these key molecules is altered under different physiological conditions. We will now examine the role of fatty acid metabolism in two such conditions: diabetes and starvation.
 CLINICAL INSIGHT
CLINICAL INSIGHTDiabetes Can Lead to a Life-Threatening Excess of Ketone-Body Production
Although ketone bodies are a normal fuel, excess amounts of these acids can be dangerous. Excess production can result from an imbalance in the metabolism of carbohydrates and fatty acids, as seen in the disease diabetes mellitus, also known as diabetes, a condition characterized by the absence of insulin or resistance to insulin.
Why does the disruption of insulin function result in disease? Insulin has two major roles in regulating metabolism. First, insulin normally stimulates the absorption of glucose by the liver. If this absorption does not take place, oxaloacetate cannot be produced to react with acetyl CoA, the product of fatty acid degradation. Recall that animals synthesize oxaloacetate from pyruvate, a product of the glycolytic processing of glucose. Indeed, in diabetics, oxaloacetate from the citric acid cycle is actually consumed to form glucose through gluconeogenesis. Second, insulin normally curtails fatty acid mobilization by adipose tissue. In the absence of functional insulin, fatty acids are released and large amounts of acetyl CoA are consequently produced by β oxidation. However, much of the acetyl CoA cannot enter the citric acid cycle, because of insufficient oxaloacetate for condensation. A striking feature of diabetes is a shift of fuel usage from carbohydrates to fats; glucose, more abundant than ever, is left unused in the bloodstream.
The liver also releases large amounts of ketone bodies into the blood because it is degrading fatty acids but lacks the glucose required to replenish the citric acid cycle (Figure 27.12). Ketone bodies are moderately strong acids, and the result of their overabundance is severe acidosis because of the inability of the kidneys to maintain acid–
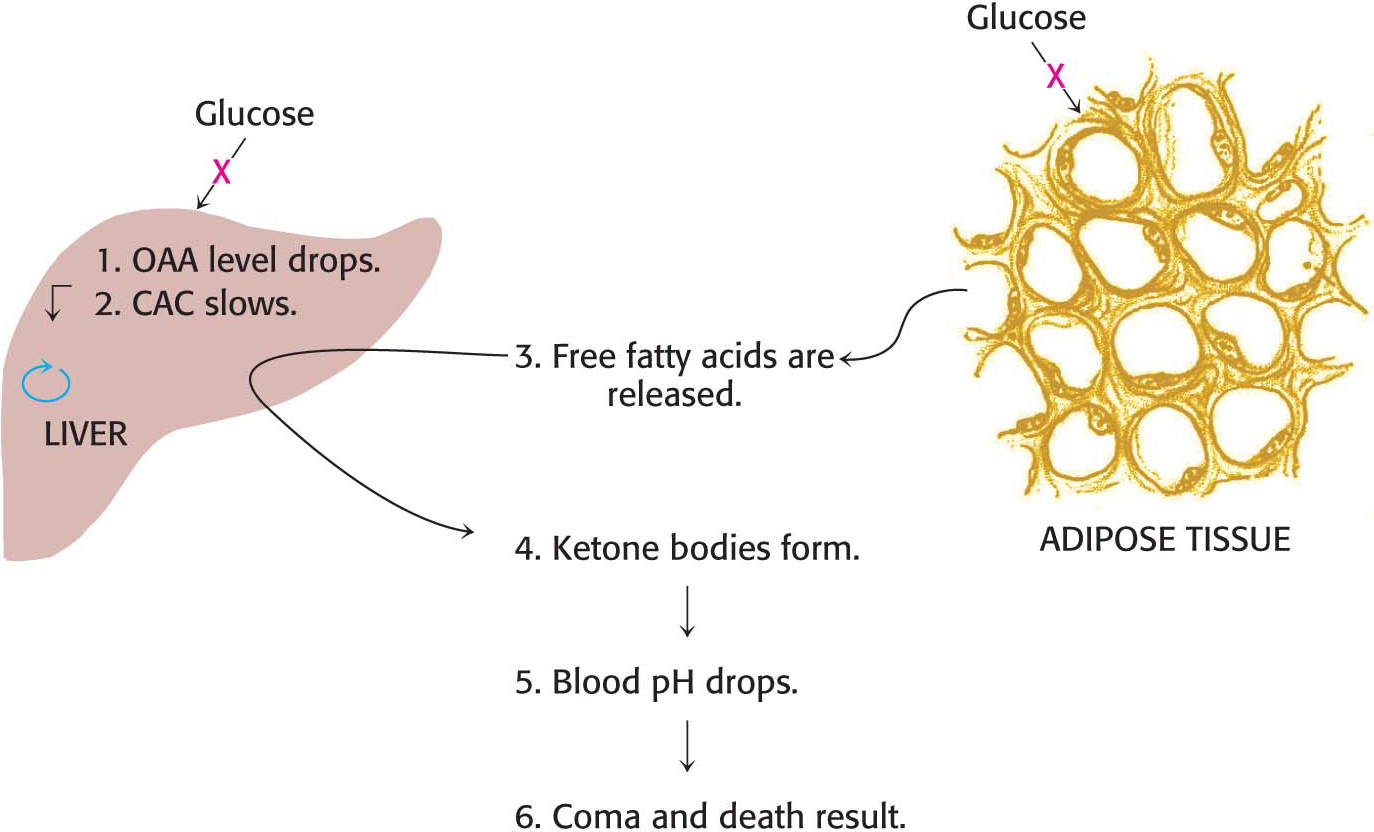
 CLINICAL INSIGHT
CLINICAL INSIGHTKetone Bodies Are a Crucial Fuel Source During Starvation
For some people, the use of ketone bodies as fuel is a matter of life or death. The bodies of starving people automatically resort to ketone bodies as a primary fuel source. Why does this adaptation take place? Let’s consider the biochemical changes that take place in the course of prolonged fasting. A typical well-
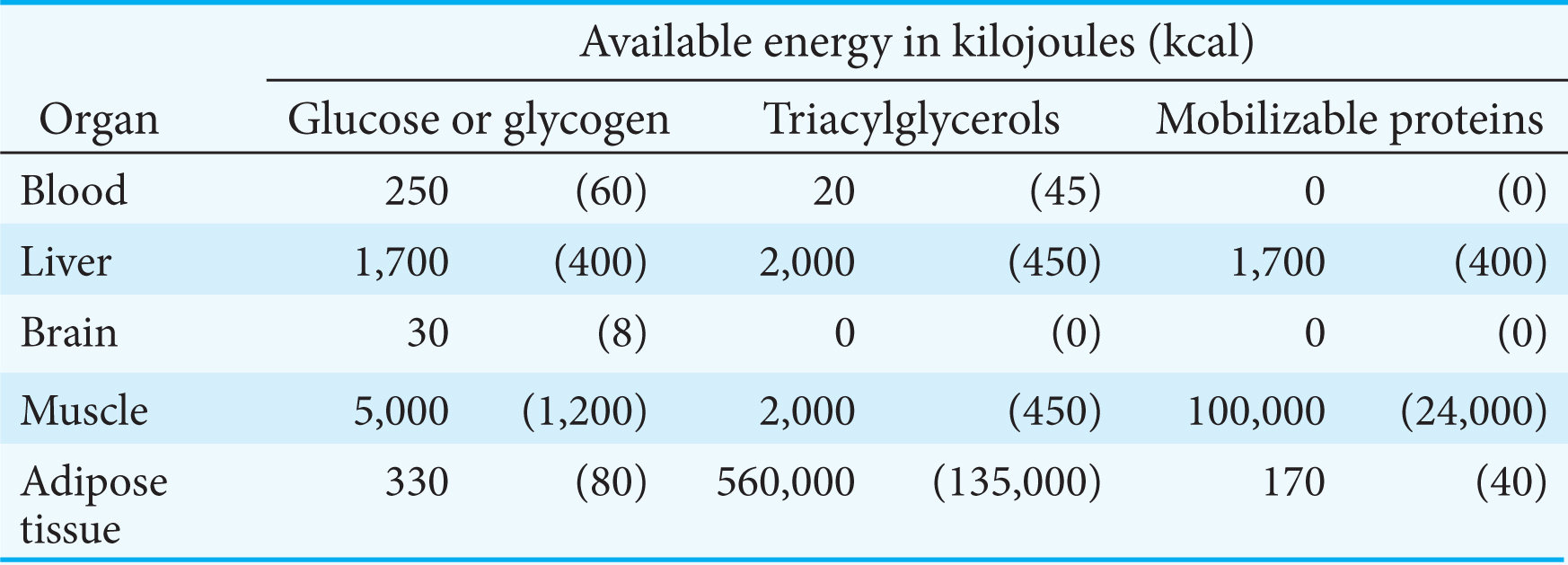
Thus, the second priority of metabolism in starvation is to preserve muscle protein by shifting the fuel being used from glucose to fatty acids and ketone bodies, particularly in organs that normally rely on glucose (Figure 27.13). How is the loss of muscle protein curtailed? First of all, muscle—
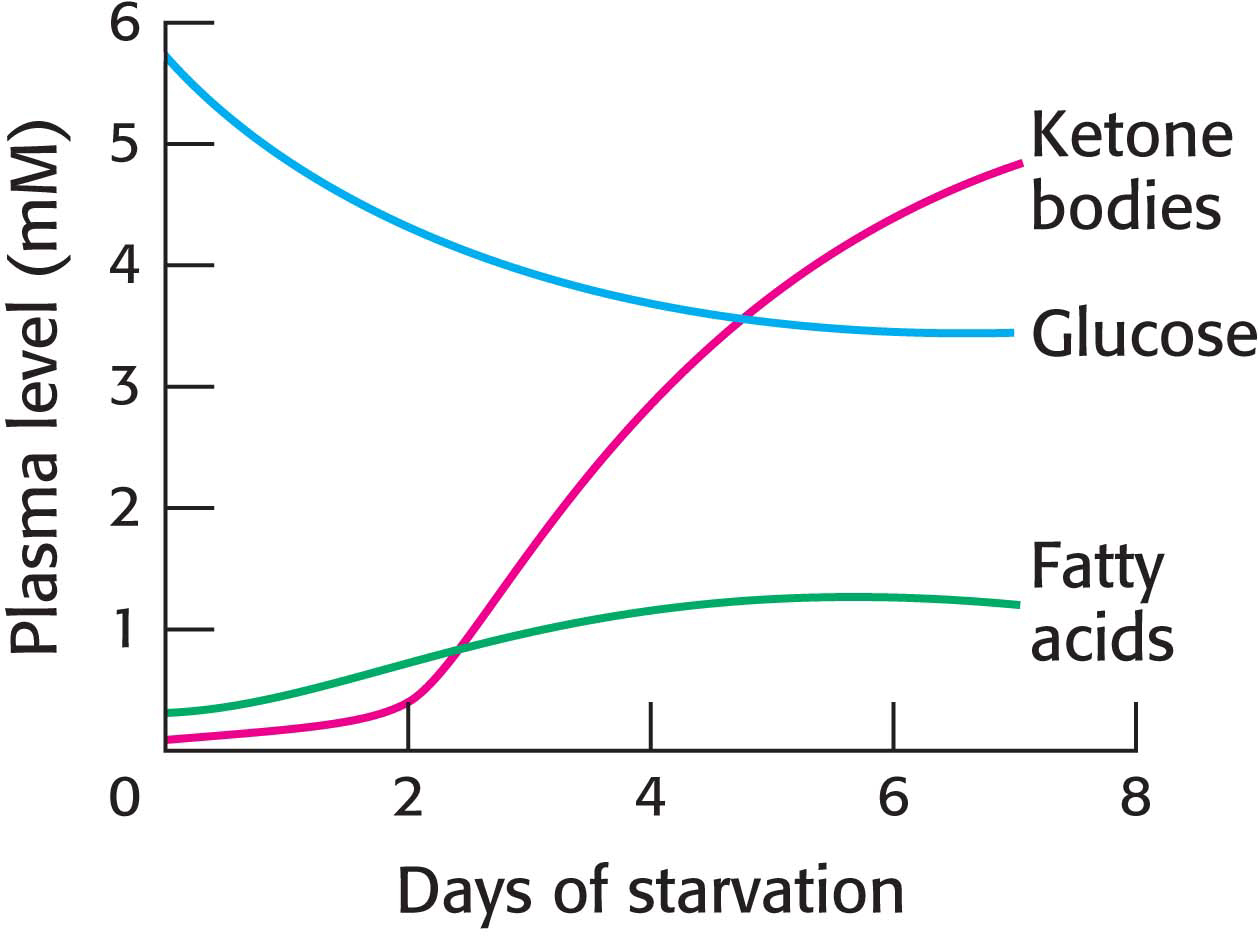
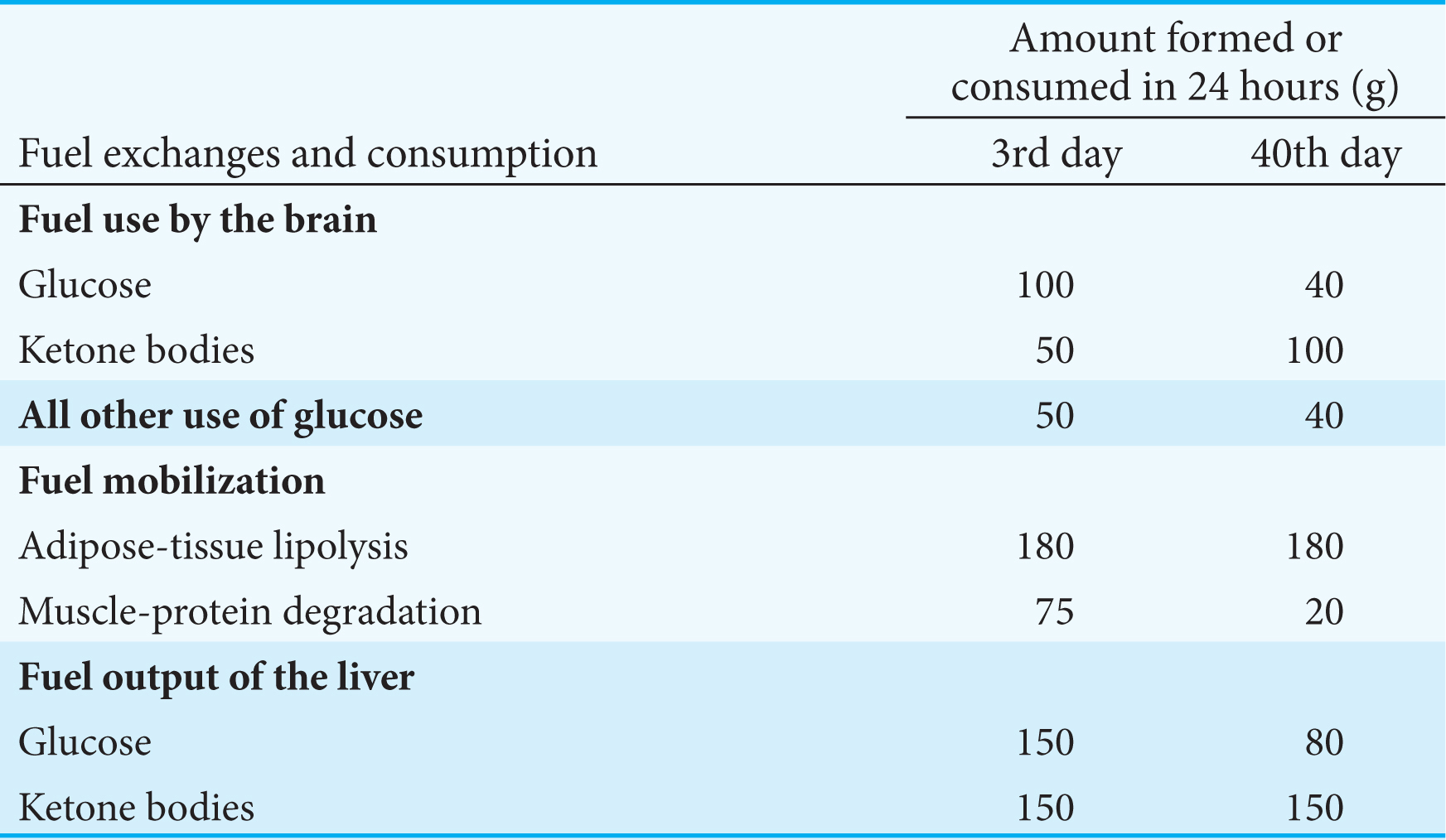
After several weeks of starvation, ketone bodies become the major fuel of the brain. Only 40 g of glucose is then needed per day for the brain, compared with about 120 g in the first day of starvation. The effective conversion of fatty acids into ketone bodies by the liver and their use by the brain markedly diminishes the need for glucose. Hence, less muscle is degraded than in the first days of starvation. The breakdown of 20 g of muscle daily compared with 75 g early in starvation is crucial for survival. A person’s survival time is mainly determined by the size of the triacylglycerol depot.
What happens when the lipid stores are gone? The only source of fuel that remains is protein. Protein degradation accelerates, and death inevitably results from a loss of heart, liver, or kidney function.
 CLINICAL INSIGHT
CLINICAL INSIGHTSome Fatty Acids May Contribute to the Development of Pathological Conditions
Certain polyunsaturated fatty acids are essential for life, serving as precursors to various signal molecules. Vegetable oils, used commonly in food preparation, are rich in polyunsaturated fatty acids. However, polyunsaturated fatty acids are unstable and are readily oxidized. This tendency to become rancid reduces their shelf life and renders them undesirable for cooking. To circumvent this problem, polyunsaturated fatty acids are hydrogenated, converting them to saturated and trans unsaturated fatty acids (popularly known as “trans fat”), a variety of fat that is rare in nature. Epidemiological evidence suggests that consumption of large amounts of saturated fatty acids and trans fat promotes obesity, type 2 diabetes, and atherosclerosis. The mechanism by which these fats exert these effects is under active investigation. Some evidence suggests that they promote an inflammatory response and may mute the action of insulin and other hormones.