PROBLEMS
Question 27.1
1. Stages of processing. What are the three stages of triacylglycerol utilization? ✓ 1
Question 27.2
2. Control matters. Outline the control of triacylglycerol mobilization. ✓ 1
Question 27.3
3. Forms of energy. The partial reactions leading to the synthesis of acyl CoA (equations 1 and 2, Section 27.1) are freely reversible. The equilibrium constant for the sum of these reactions is close to 1, meaning that the energy levels of the reactants and products are about equal, even though a molecule of ATP has been hydrolyzed. Explain why these reactions are readily reversible. ✓ 1
Question 27.4
4. In its entirety. Write the complete reaction for fatty acid activation. ✓ 1
Question 27.5
5. Activation fee. The reaction for the activation of fatty acids before degradation is
This reaction is quite favorable because the equivalent of two molecules of ATP is hydrolyzed. Explain why, from a biochemical bookkeeping point of view, the equivalent of two molecules of ATP is used despite the fact that the left side of the equation has only one molecule of ATP. ✓ 1
Question 27.6
6. Repeating reactions. What are the recurring reactions of the oxidation of saturated fatty acids? ✓ 1
Question 27.7
7. Like Simon and Garfunkel. Match each term with its description. ✓ 1
Triacylglycerol Perilipin Adipose triglyceride lipase Glucagon Acyl CoA synthetase Carnitine β-Oxidation pathway Enoyl CoA isomerase 2,4- Methylmalonyl CoA mutase Ketone body | Lipid- Reduces 2,4- Activates fatty acids for degradation Stimulates lipolysis Converts a cis-Δ3 double bond into a trans- Means by which fatty acids are degraded Requires vitamin B12 Acetoacetate The enzyme that initiates lipid degradation Required for entry into mitochondria Storage form of fats |
Question 27.8
8. Proper sequence. Place the following list of reactions or relevant locations in the β oxidation of fatty acids in the proper order. ✓ 1
(a) Reaction with carnitine
(b) Fatty acid in the cytoplasm
(c) Activation of fatty acid by joining to CoA
(d) Hydration
(e) NAD+-linked oxidation
(f) Thiolysis
(g) Acyl CoA in mitochondrion
(h) FAD-
Question 27.9
9. Too tired to exercise. Explain why people with a hereditary deficiency of carnitine acyltransferase II have muscle weakness. Why are the symptoms more severe during fasting? ✓ 1
Question 27.10
10. A phantom acetyl CoA? In the equation for fatty acid degradation shown here, only seven molecules of CoA are required to yield eight molecules of acetyl CoA. How is this difference possible? ✓ 1
Question 27.11
11. Comparing yields. Compare the ATP yields from palmitic acid and palmitoleic acid. ✓ 1
Question 27.12
12. Counting ATPs 1. What is the ATP yield for the complete oxidation of C17 (heptadecanoic) fatty acid? Assume that the propionyl CoA ultimately yields oxaloacetate in the citric acid cycle. ✓ 1
Question 27.13
13. Sweet temptation. Stearic acid is a C18 fatty acid component of chocolate. Suppose you had a depressing day and decided to settle matters by gorging on chocolate. How much ATP would you derive from the complete oxidation of stearic acid to CO2? ✓ 1
Question 27.14
14. Buff and lean. It has been suggested that if you are interested in losing body fat, the best time to do strenuous aerobic exercise is in the morning immediately after waking up; that is, after fasting. Don’t eat breakfast before exercising, but have a cup of caffeinated coffee. Caffeine is an inhibitor of cAMP phosphodiesterase. Explain why this suggestion might work biochemically. ✓ 1
Question 27.15
15. The best storage form. Compare the ATP yield from the complete oxidation of glucose, a six-
Question 27.16
16. From fatty acid to ketone body. Write a balanced equation for the conversion of stearate into acetoacetate. ✓ 2
Question 27.17
17. Generous, but not to a fault. Liver is the primary site of ketone-
Question 27.18
18. Counting ATPs 2. How much energy is attained with the complete oxidation of the ketone body d-3-
Question 27.19
19. Another view. Why might someone argue that the answer to problem 18 is wrong? (Consider the uses of succinyl CoA.) ✓ 2
Question 27.20
20. An accurate adage. An old biochemistry adage is that fats burn in the flame of carbohydrates. What is the molecular basis of this adage? ✓ 2
Question 27.21
21. Missing acyl CoA dehydrogenases. A number of genetic deficiencies in acyl CoA dehydrogenases have been described. A deficiency in acyl CoA dehydrogenase presents itself early in life or after a period of fasting. Symptoms include vomiting, lethargy, and, sometimes, coma. Not only are blood levels of glucose low (hypoglycemia), but also starvation-
Question 27.22
22. Missing ingredient. Why are liver cells not capable of using ketone bodies as a fuel? ✓ 2
Question 27.23
23. Finding triacylglycerols in all the wrong places. Insulin-
Chapter Integration Problems
Question 27.24
24. Leaner times might follow. Why can’t animals convert fats into glucose? Why are plants capable of such a conversion?
Question 27.25
25. Losing protein. What is the purpose of protein degradation during the initial stages of starvation?
Question 27.26
26. Stop losing protein. How is the loss of muscle protein delayed during starvation?
Question 27.27
27. After lipolysis. During fatty acid mobilization, glycerol is produced. This glycerol is not wasted. Write a balanced equation for the conversion of glycerol into pyruvate. What enzymes are required in addition to those of the glycolytic pathway?
Question 27.28
28. Remembrance of reactions past. We encountered reactions similar to the oxidation, hydration, and oxidation reactions of fatty acid degradation earlier in our study of biochemistry. What other pathway employs this set of reactions?
Question 27.29
29. Ill-
(a) How would a lack of carbohydrates affect your ability to utilize fats?
(b) What would your breath smell like?
(c) One of your best friends, after trying unsuccessfully to convince you to abandon this diet, makes you promise to consume a healthy dose of odd-
Data Interpretation Problem
Question 27.30
30. Mutant enzyme. Carnitine palmitoyl transferase I (CPTI) catalyzes the conversion of long-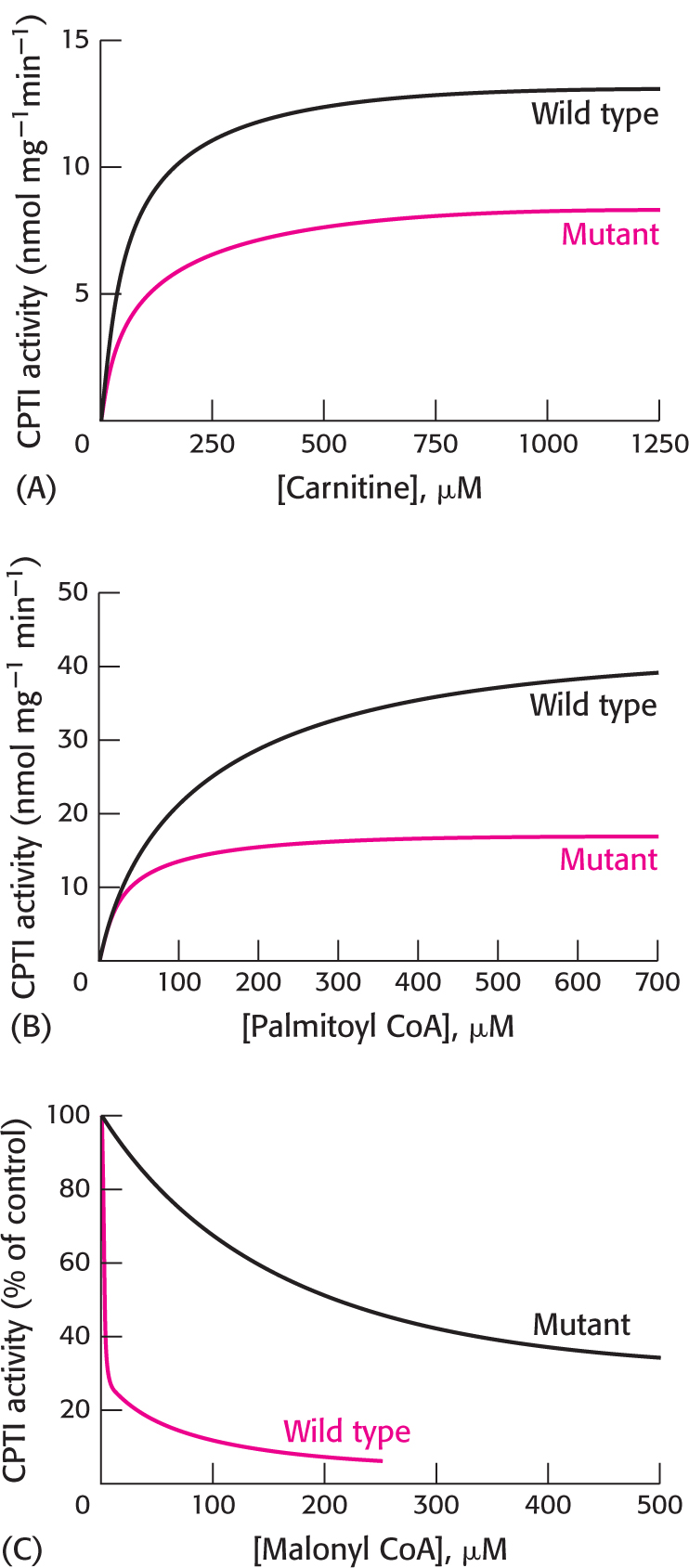
(a) What is the effect of the mutation on enzyme activity when the concentration of carnitine is varied? What are the KM and Vmax values for the wild-
(b) What is the effect when the experiment is repeated with varying concentrations of palmitoyl CoA? What are the KM and Vmax values for the wild-
(c) Graph C shows the inhibitory effect of malonyl CoA on the wild-
(d) Suppose that the concentration of palmitoyl CoA is 100 μM, that of carnitine is 100 μM, and that of malonyl CoA is 10 μM. Under these conditions, what is the most prominent effect of the mutation on the properties of the enzyme?
(e) What can you conclude about the role of glutamate 3 in carnitine acyltransferase I function?
Challenge Problems
Question 27.31
31. Refsum disease. Phytanic acid is a branched-
(a) Why does phytanic acid accumulate?
(b) What enzyme activity would you invent to prevent its accumulation?
Question 27.32
32. Sleight of hand. Animals cannot affect the net synthesis of glycogen from fatty acids. Yet, if animals are fed radioactive lipids (14C), over time, some radioactive glycogen appears. How is the appearance of radioactive glycogen possible in these animals?
Question 27.33
33. Necessary diversion. When acetyl CoA produced by β-oxidation exceeds the capacity of the citric acid cycle, ketone bodies are produced. Although acetyl CoA is not toxic, mitochondria must divert acetyl CoA to ketone bodies to keep functioning. Explain why. What would happen if ketone bodies were not generated?
Question 27.34
34. A hot diet. Tritium is a radioactive isotope of hydrogen and can be readily detected. Fully tritiated, six-
Selected Readings for this chapter can be found online at www.whfreeman.com/