
28.1 Fatty Acid Synthesis Takes Place in Three Stages
✓ 3 Explain how fatty acids are synthesized.
As is the case for fatty acid degradation, fatty acid synthesis occurs as three stages of processing:
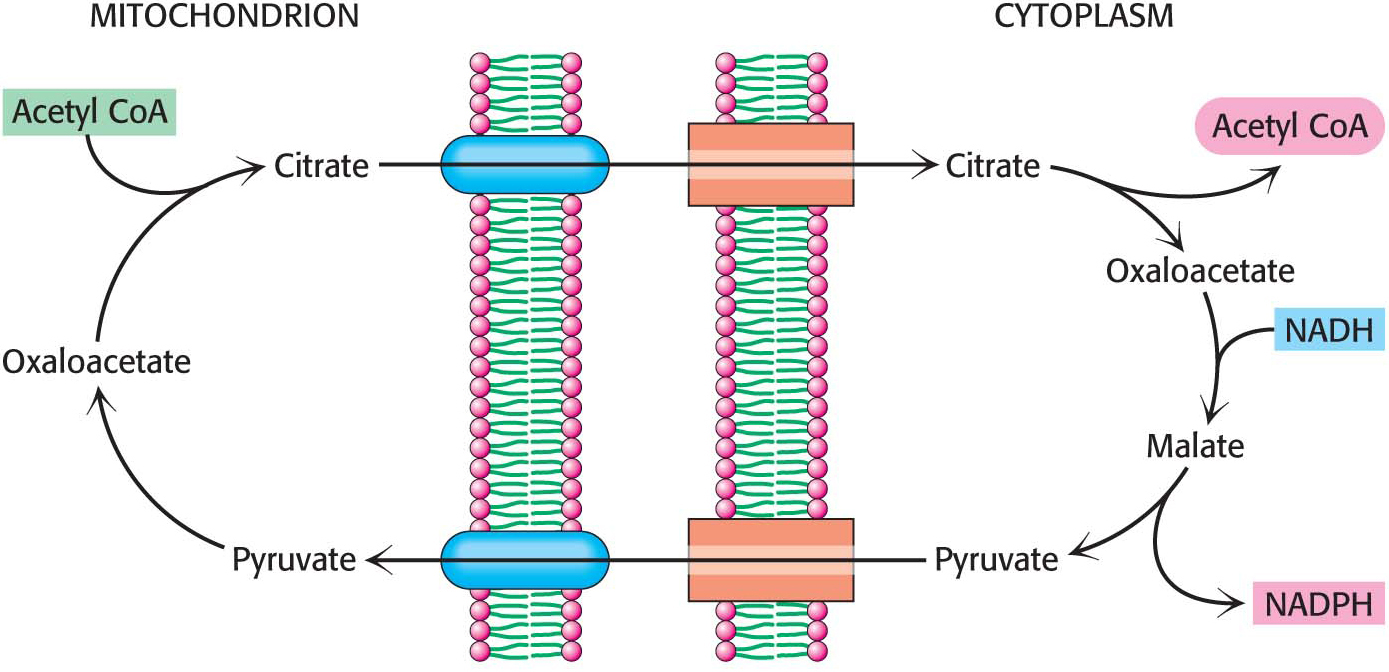
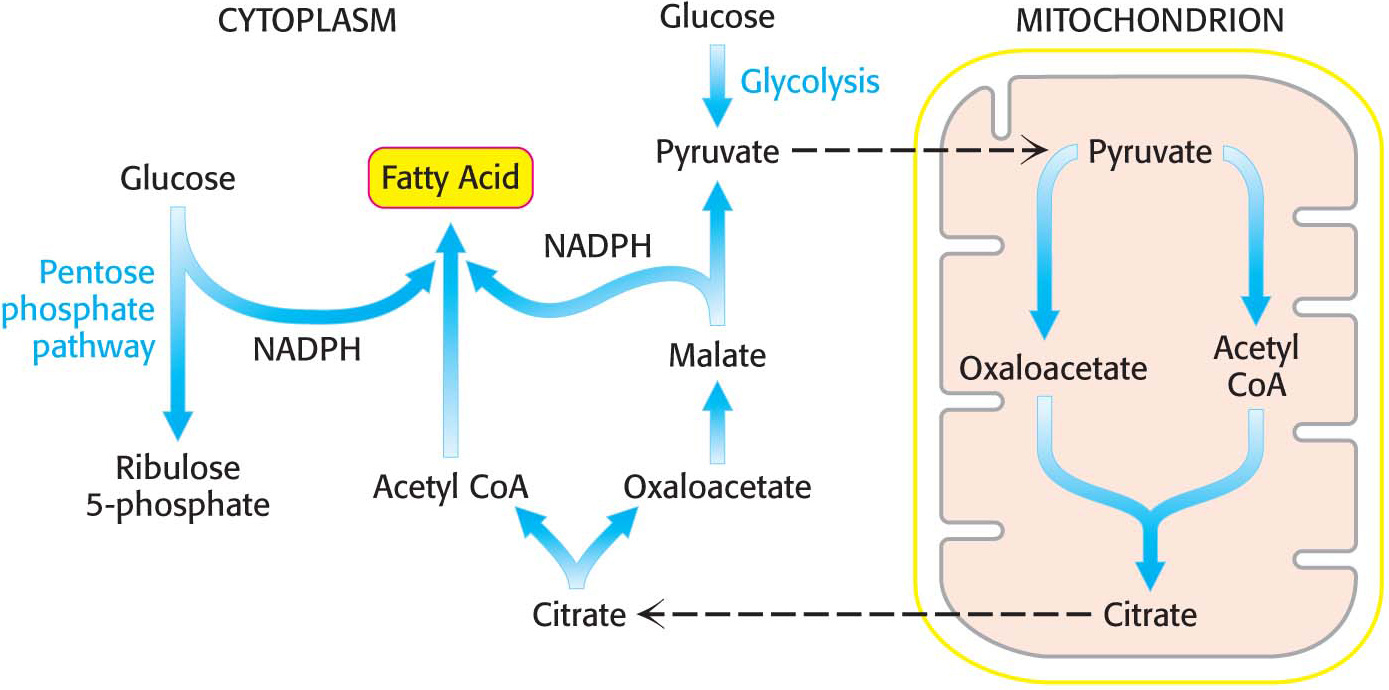
In a preparatory step, acetyl CoA is transferred from mitochondria, where it is produced, to the cytoplasm, the site of fatty acid synthesis. Acetyl CoA is transported in the form of citrate, which is cleaved to yield acetyl CoA and oxaloacetate.
Page 508Fatty acid synthesis begins in the cytoplasm with the activation of acetyl CoA, in a two-
step reaction, to malonyl CoA. The reaction intermediates are attached to an acyl carrier protein, which serves as the molecular foundation for the fatty acid being constructed. Fatty acid is synthesized, two carbon atoms at a time, in a five-
step elongation cycle.
Citrate Carries Acetyl Groups from Mitochondria to the Cytoplasm
The first biochemical hurdle in fatty acid synthesis is that synthesis takes place in the cytoplasm, but acetyl CoA, the raw material for fatty acid synthesis, is formed in mitochondria. The mitochondria are not readily permeable to acetyl CoA. How are acetyl CoA molecules transferred to the cytoplasm? The problem is solved by the transport of acetyl CoA out of the mitochondria in the form of citrate. Citrate is formed in the mitochondrial matrix by the condensation of acetyl CoA with oxaloacetate (Figure 28.1). This reaction begins the citric acid cycle when energy is required. When the energy needs of a cell have been met, citrate is relocated to the cytoplasm by a transport protein, where it is cleaved by ATP-
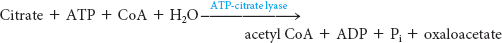
This reaction occurs in three steps: (1) the formation of a phospho-
In addition to being a precursor for fatty acid synthesis, citrate serves as a signal molecule. It inhibits phosphofructokinase, which controls the rate of glycolysis. Citrate in the cytoplasm indicates an energy-
Several Sources Supply NADPH for Fatty Acid Synthesis
The synthesis of palmitate requires 14 molecules of NADPH as well as the expenditure of ATP. Some of the reducing power is generated when oxaloacetate, formed in the transfer of acetyl groups to the cytoplasm, is returned to the mitochondria. The inner mitochondrial membrane is impermeable to oxaloacetate. Hence, a series of bypass reactions are needed. First, oxaloacetate is reduced to malate by NADH. This reaction is catalyzed by a cytoplasmic malate dehydrogenase:

Second, malate is oxidatively decarboxylated by an NADP+-linked malate enzyme (also called malic enzyme).
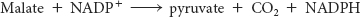
The pyruvate formed in this reaction readily enters mitochondria, where it is carboxylated to oxaloacetate by pyruvate carboxylase:

The sum of these three reactions is
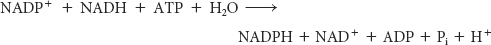
Thus, one molecule of NADPH is generated for each molecule of acetyl CoA that is transferred from mitochondria to the cytoplasm. Hence, eight molecules of NADPH are formed when eight molecules of acetyl CoA are transferred to the cytoplasm for the synthesis of palmitate. The additional six molecules of NADPH required for the synthesis of palmitate come from the pentose phosphate pathway (Chapter 26).
The accumulation of the precursors for fatty acid synthesis is a wonderful example of the coordinated use of multiple pathways. The citric acid cycle, the transport of citrate from mitochondria, and the pentose phosphate pathway provide the carbon atoms and reducing power, whereas glycolysis and oxidative phosphorylation provide the ATP to meet the needs for fatty acid synthesis (Figure 28.2).
The Formation of Malonyl CoA Is the Committed Step in Fatty Acid Synthesis
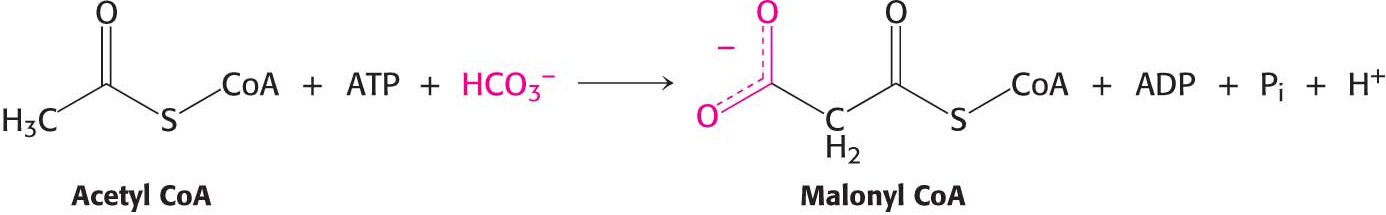
Like the synthesis of all biopolymers, fatty acid synthesis requires an activation step. Fatty acid synthesis starts with the carboxylation of acetyl CoA to malonyl CoA, the activated form of acetyl CoA. This reaction is catalyzed by acetyl CoA carboxylase 1, a regulatory enzyme in fatty acid metabolism. As we will see shortly, malonyl CoA is the actual carbon donor for all but two of the carbon atoms of palmitic acid.
NUTRITION FACTS

Acetyl CoA is combined with HCO3−, the form of CO2 in aqueous solutions. This irreversible reaction is the committed step in fatty acid synthesis:
The synthesis of malonyl CoA is a two-
Acetyl CoA carboxylase contains a biotin prosthetic group. In the first step, a carboxybiotin intermediate is formed at the expense of the hydrolysis of a molecule of ATP:

The activated CO2 group is then transferred to acetyl CoA to form malonyl CoA:

Acetyl CoA carboxylase 2, an isozyme of carboxylase 1 located in the mitochondria, is the essential regulatory enzyme for fatty acid degradation. Mechanistically, it functions like carboxylase 1.
Fatty Acid Synthesis Consists of a Series of Condensation, Reduction, Dehydration, and Reduction Reactions
The enzyme system that catalyzes the synthesis of saturated long-
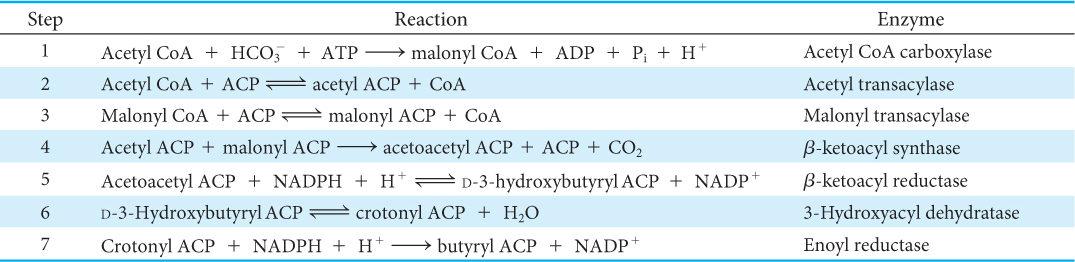
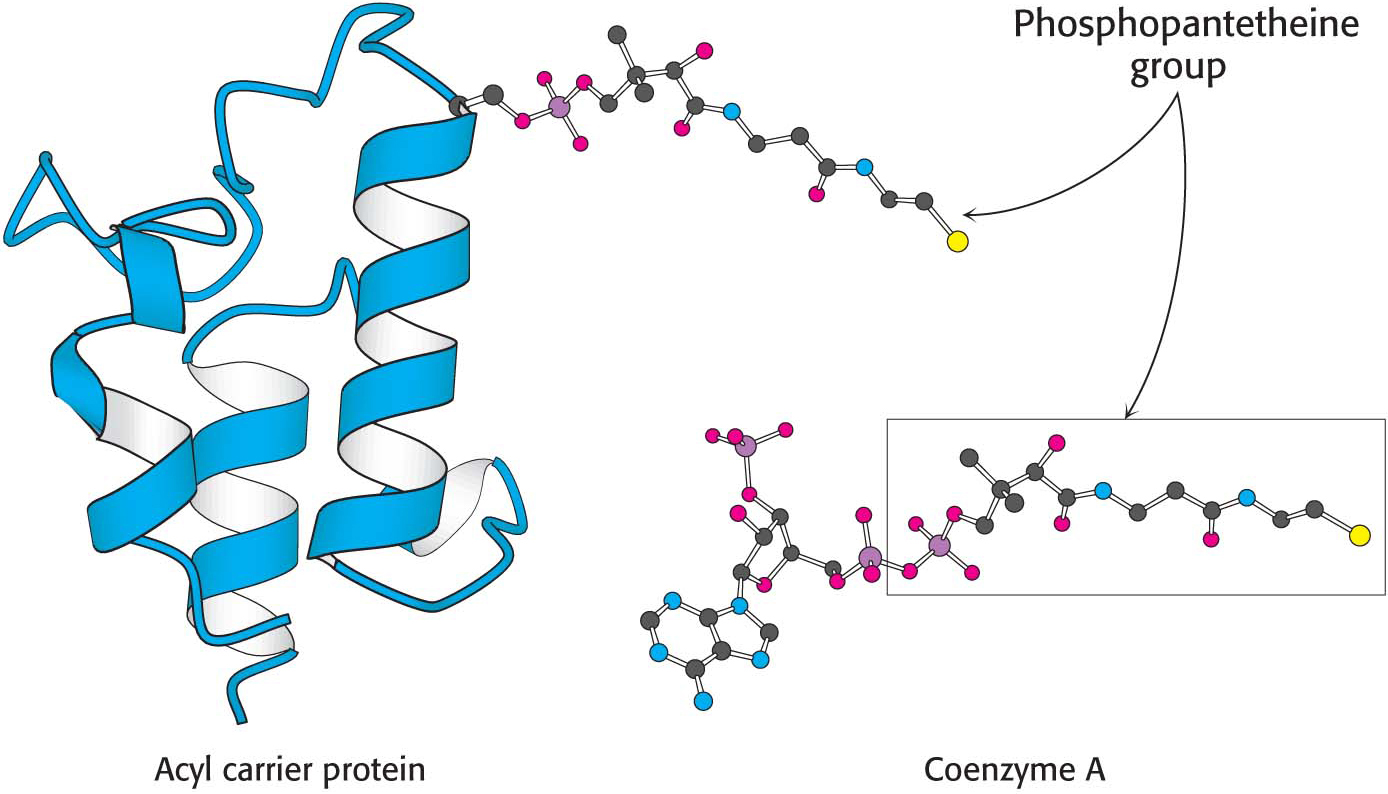
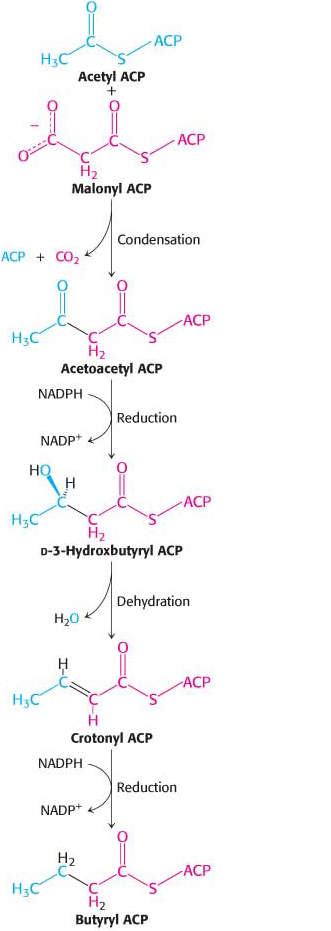
Acyl carrier protein, a single polypeptide chain of 77 residues, can be regarded as a giant prosthetic group, a “macro CoA.” Acetyl transacylase and malonyl transacylase catalyze the formation of acetyl ACP and malonyl ACP, respectively:
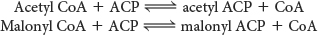
The synthesis of fatty acids with an odd number of carbon atoms starts with propionyl ACP, which is formed from propionyl CoA by acetyl transacylase.
Acetyl ACP and malonyl ACP react to form acetoacetyl ACP (Figure 28.4). β-Ketoacyl synthase, also called the condensing enzyme, catalyzes this condensation reaction.
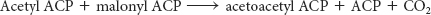
In the condensation reaction, a four-
DID YOU KNOW?
Triclosan binds to the enoyl substrate-
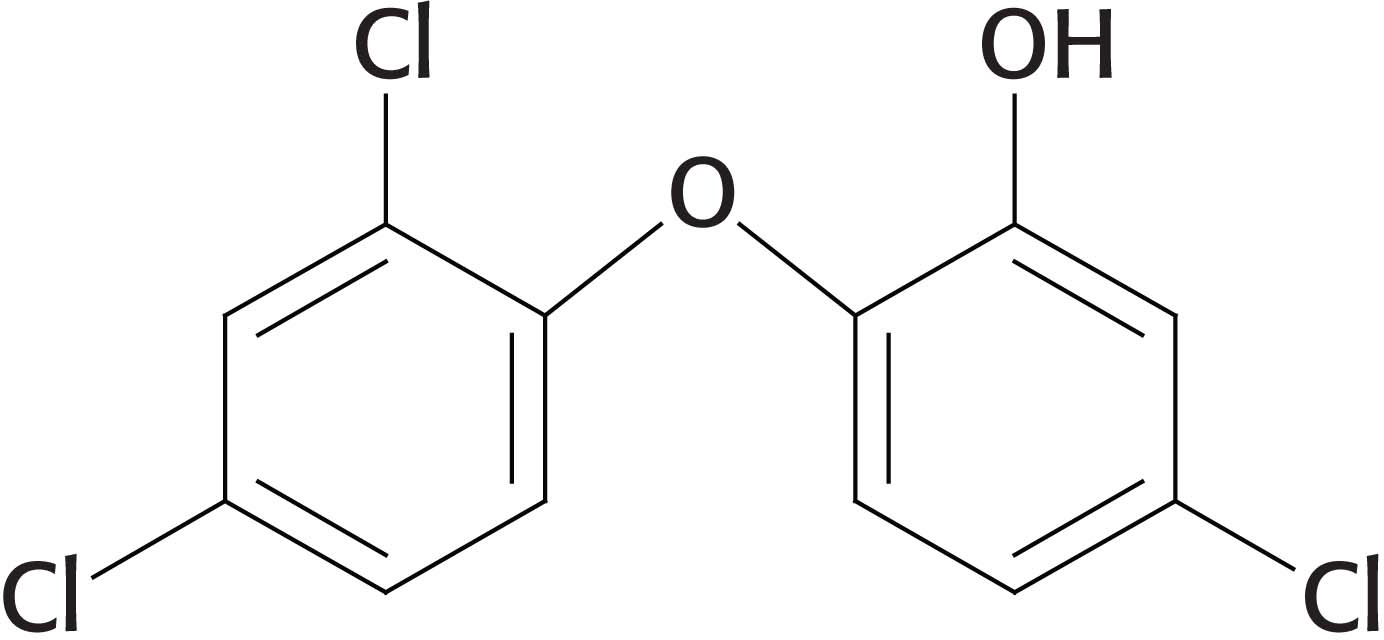
The next three steps in fatty acid synthesis reduce the keto group (shown in the margin) at C-
In the second round of fatty acid synthesis, butyryl ACP condenses with another malonyl ACP to form a C6-β-ketoacyl ACP. This reaction is like the one in the first round, in which acetyl ACP condenses with malonyl ACP to form a C4-β-ketoacyl ACP. Reduction, dehydration, and a second reduction convert the C6-β-ketoacyl ACP into a C6-acyl ACP, which is ready for a third round of elongation. The elongation cycles continue until C16-acyl ACP is formed. This intermediate is a good substrate for a thioesterase that hydrolyzes C16-acyl ACP to release the fatty acid chain from the carrier protein. Because it acts selectively on C16-acyl ACP, the thioesterase acts as a ruler to determine fatty acid chain length. The synthesis of longer-
The Synthesis of Palmitate Requires 8 Molecules of Acetyl CoA, 14 Molecules of NADPH, and 7 Molecules of ATP
Now that we have seen all of the individual reactions of fatty acid synthesis, let us determine the overall reaction for the synthesis of the C16 fatty acid palmitate. The stoichiometry of the synthesis of palmitate is
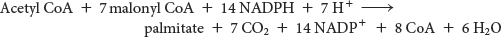
The equation for the synthesis of the malonyl CoA used in the preceding reaction is
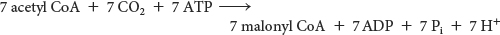
Hence, the overall stoichiometry for the synthesis of palmitate is
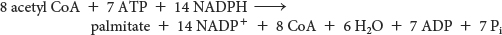
Fatty Acids Are Synthesized by a Multifunctional Enzyme Complex in Animals
Although the basic biochemical reactions in fatty acid synthesis are similar in E. coli and eukaryotes, the structure of the synthase varies considerably. The component enzymes of animal fatty acid synthases, in contrast with those of E. coli and plants, are linked in a large polypeptide chain.
The structure of a large part of the mammalian fatty acid synthase has recently been determined, with the acyl carrier protein and thioesterase remaining to be resolved. The enzyme is a dimer of identical 270-
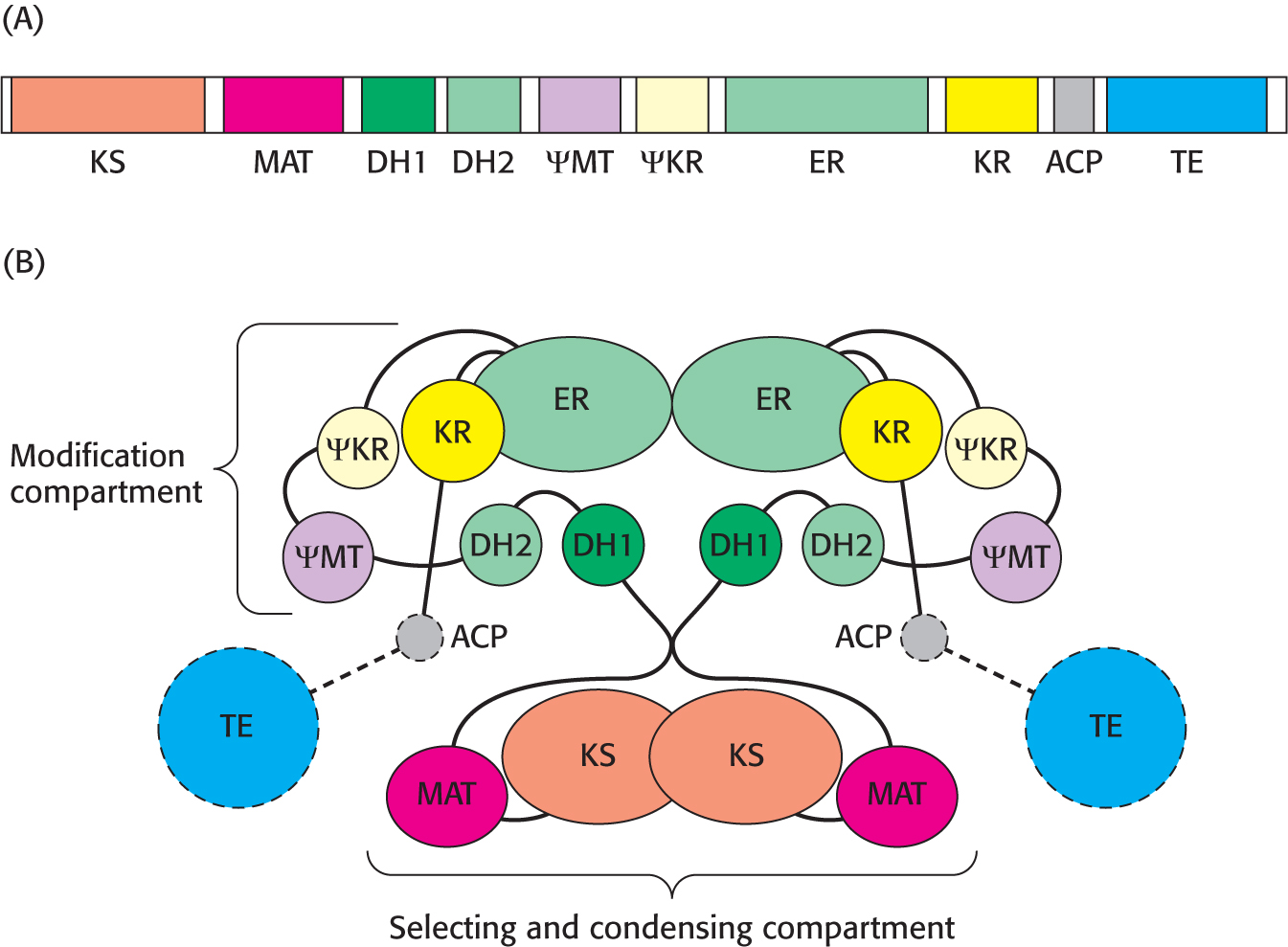
The two component chains interact such that the enzyme activities are partitioned into two distinct compartments (Figure 28.5B). The selecting and condensing compartment binds the acetyl and malonyl substrates and condenses them to form the growing chain. Interestingly, the mammalian fatty acid synthase has one active site, malonyl-
QUICK QUIZ 1
What are the raw materials for fatty acid synthesis, and how are they obtained?
Acetyl CoA is the basic substrate for fatty acid synthesis. It is transported out of mitochondria in the form of citrate. After the formation of acetyl CoA, the resulting pyruvate is transported back into the mitochondria with a concomitant formation of NADPH, the reducing power for fatty acid synthesis. Additional NADPH can be generated by the pentose phosphate pathway. Malonyl CoA, the ultimate substrate for fatty acid synthesis is formed by the carboxylation of acetyl CoA.
Many eukaryotic multienzyme complexes are multifunctional proteins in which different enzymes are linked covalently. An advantage of this arrangement is that the synthetic activity of different enzymes is coordinated. In addition, intermediates can be efficiently handed from one active site to another without leaving the assembly.
 CLINICAL INSIGHT
CLINICAL INSIGHTFatty Acid Metabolism Is Altered in Tumor Cells
We have previously seen that cancer cells alter glucose metabolism to meet the needs of rapid cell growth. Cancer cells must also increase fatty acid synthesis for use as signal molecules as well as for incorporation into membrane phospholipids. Many of the enzymes of fatty acid synthesis are overexpressed in most human cancers, and this expression is correlated with tumor malignancy. Recall that normal cells do little de novo fatty acid synthesis, relying instead on dietary intake to meet their fatty needs.
The dependence of de novo fatty acid synthesis provides possible therapeutic targets to inhibit cancer cell growth. Inhibition of β-ketoacyl ACP synthase, the enzyme that catalyzes the condensation step of fatty acid synthesis, does indeed inhibit phospholipid synthesis and subsequent cell growth in some cancers, apparently by inducing programmed cell death (apoptosis). However, another startling observation was made: mice treated with inhibitors of the condensing enzyme showed remarkable weight loss because they ate less. Thus, fatty acid synthase inhibitors are exciting candidates both as antitumor and as antiobesity drugs.
Acetyl CoA carboxylase is also being investigated as a possible target for inhibiting cancer cell growth. Inhibition of the carboxylase in prostate and breast cancer cell lines induces apoptosis in the cancer cells, and yet is without effect in normal cells (problem 26). Understanding the alteration of fatty acid metabolism in cancer cells is a developing area of research that holds promise of generating new cancer therapies.
 CLINICAL INSIGHT
CLINICAL INSIGHTA Small Fatty Acid That Causes Big Problems
γ-Hydroxybutyric acid (GHB) is a short-
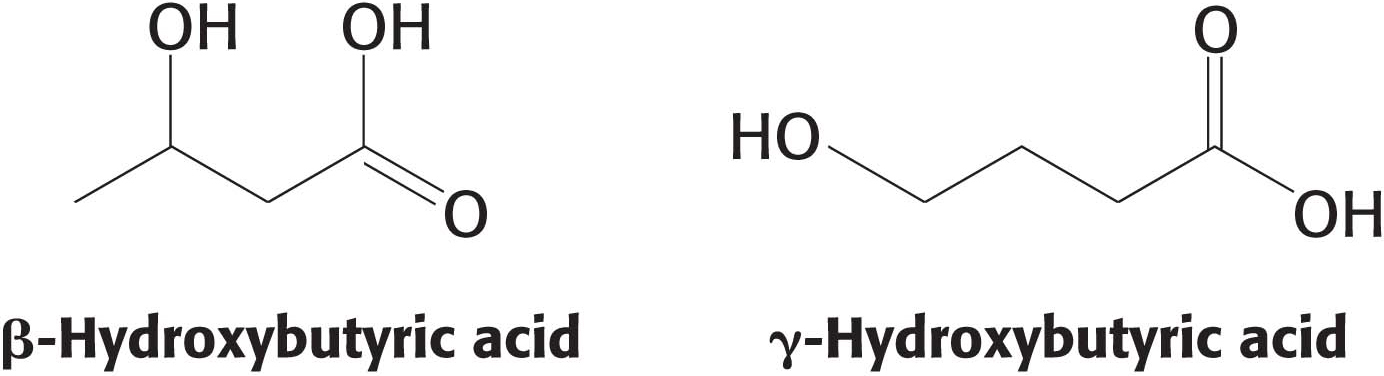
This small chemical difference of where the alcohol group (—OH) is located has a great effect on the actions of these two chemicals. Small amounts of GHB are found in the brain, where it is thought to be a neurotransmitter. GHB has been used clinically as an anesthetic and for the treatment of narcolepsy and alcoholism, but it entered recreational drug use when body builders found that it stimulated the release of growth hormone. It became a popular drug because of claims that it reduces inhibition and heightens sexual awareness, and it is notorious as a date-