
28.3 Acetyl CoA Carboxylase Is a Key Regulator of Fatty Acid Metabolism
✓ 4 Explain how fatty acid metabolism is regulated.
Fatty acid metabolism is stringently controlled so that synthesis and degradation are highly responsive to physiological needs. Fatty acid synthesis is maximal when carbohydrates and energy are plentiful and when fatty acids are scarce. Acetyl CoA carboxylase 1 and 2 play essential roles in regulating fatty acid synthesis and degradation. Recall that acetyl CoA carboxylase 1 catalyzes the committed step in fatty acid synthesis: the production of malonyl CoA (the activated two-
Acetyl CoA Carboxylase Is Regulated by Conditions in the Cell
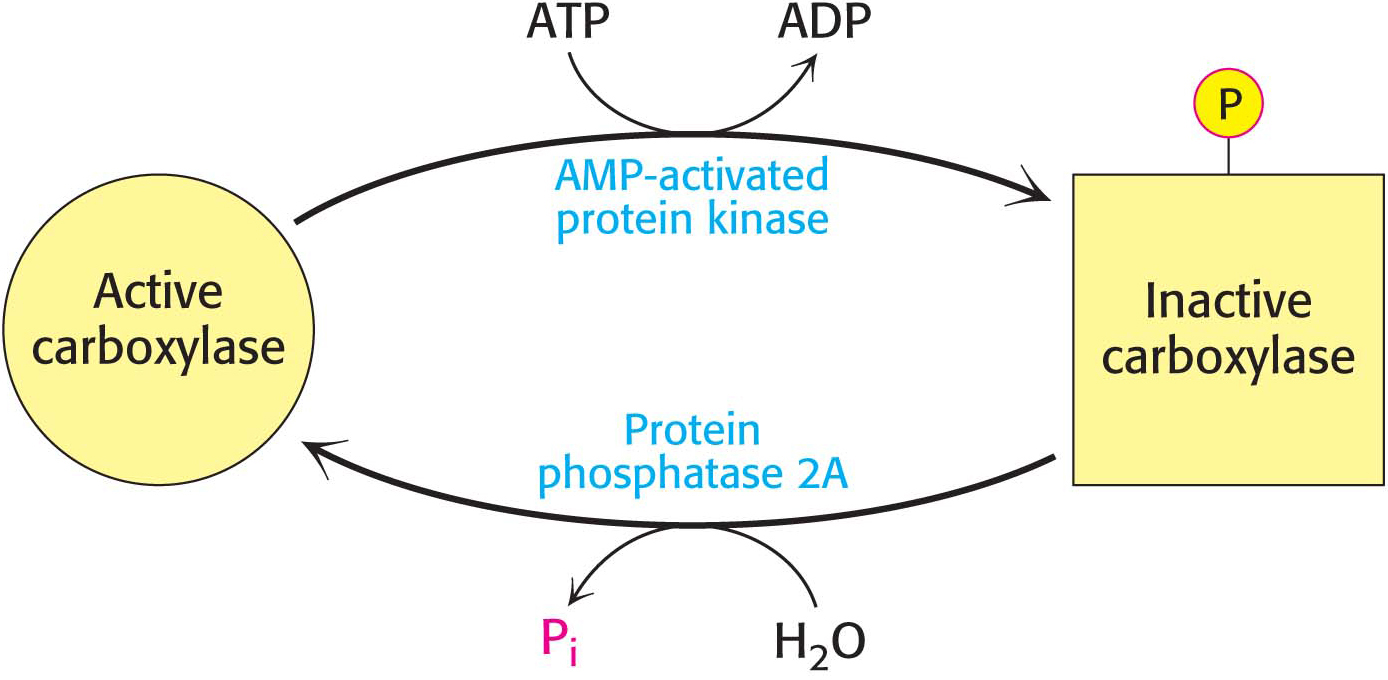
Acetyl CoA carboxylase 1 responds to changes in its immediate environment, and is switched off by phosphorylation and activated by dephosphorylation (Figure 28.8). AMP-
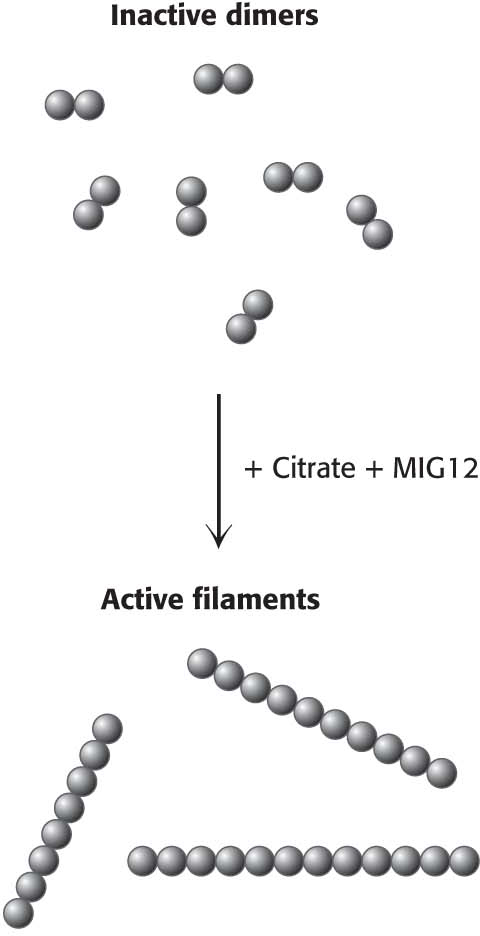
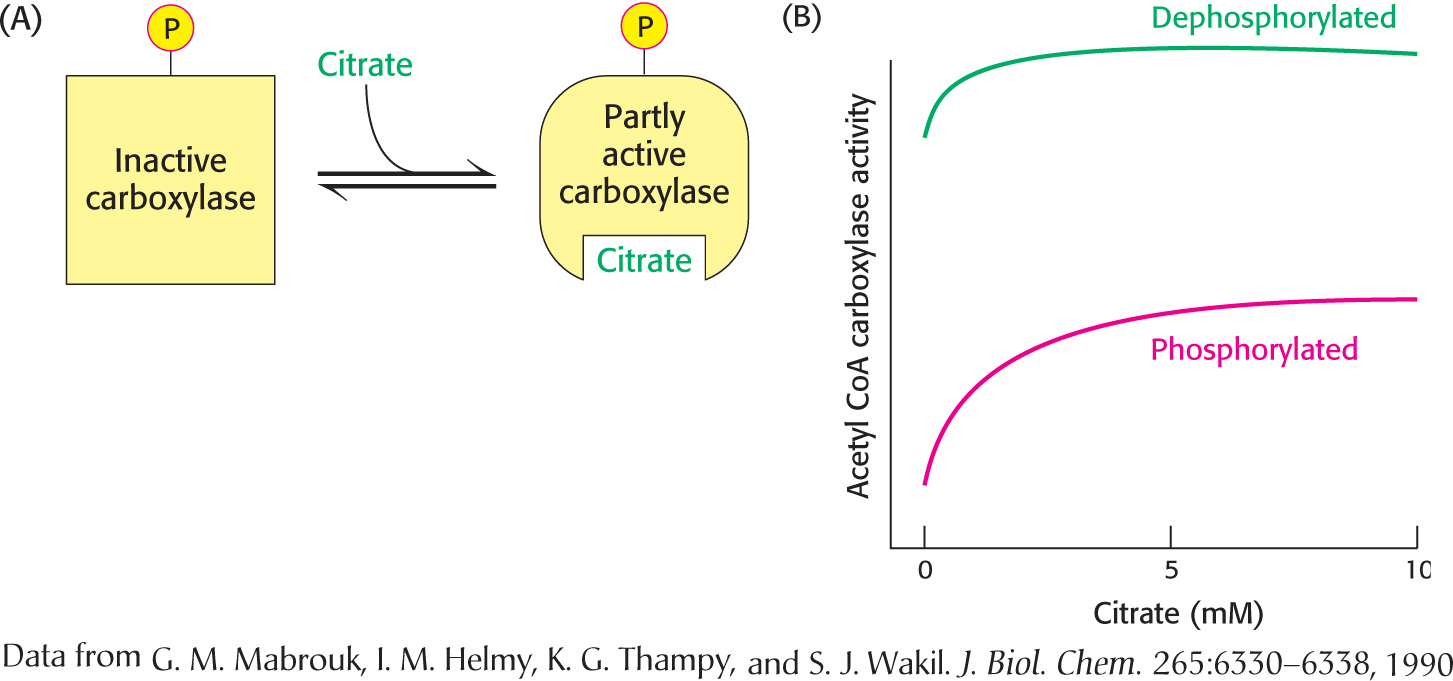
The carboxylase is also allosterically stimulated by citrate. The level of citrate is high when both acetyl CoA and ATP are abundant, signifying that raw materials and energy are available for fatty acid synthesis. Citrate acts in an unusual manner on inactive acetyl CoA carboxylase, which exists as inactive isolated dimers. Citrate facilitates the polymerization of the dimers into active filaments (Figure 28.9). However, polymerization by citrate alone requires much higher concentrations than are normally present in the cell. Under physiological conditions, citrate-
The isozyme, acetyl CoA carboxylase 2, located in the mitochondria, plays a role in the regulation of fatty acid degradation. Malonyl CoA, the product of the carboxylase reaction, is present at a high level when fuel molecules are abundant. Mitochondrial malonyl CoA inhibits carnitine acyltransferase I, preventing the entry of fatty acyl CoAs into the mitochondrial matrix in times of plenty. Malonyl CoA is an especially effective inhibitor of carnitine acyltransferase I in heart and muscle, tissues that have little fatty-
Acetyl CoA Carboxylase Is Regulated by a Variety of Hormones
Carboxylase is also controlled by the hormones glucagon, epinephrine, and insulin, which indicate the overall energy status of the organism. Insulin stimulates fatty acid synthesis by activating the carboxylase 1, whereas glucagon and epinephrine have the reverse effect.
Regulation by glucagon and epinephrineConsider, as we did in Chapter 27, a person who has just awakened from a night’s sleep and begins to exercise. Glycogen stores will be low, but lipids are readily available for mobilization. The hormones glucagon and epinephrine, present under conditions of fasting and exercise, will stimulate the mobilization of fatty acids from triacylglycerols in fat cells, which will be released into the blood, and probably in muscle cells, where the fatty acids will be used immediately as fuel. These same hormones will inhibit fatty acid synthesis by inhibiting acetyl CoA carboxylase 1. Although the exact mechanism by which these hormones exert their effects is complex, the net result is to augment the inhibition by the AMP-
Regulation by insulinNow consider the situation after the exercise has ended and the exerciser has had a meal. In this case, the hormone insulin inhibits the mobilization of fatty acids and stimulates their accumulation as triacylglycerols by muscle and adipose tissue. Insulin also stimulates fatty acid synthesis by activating acetyl CoA carboxylase 1. Insulin activates the carboxylase by enhancing the phosphorylation of AMPK by Akt, which inhibits AMPK, as well as by stimulating the activity of a protein phosphatase that dephosphorylates and activates acetyl CoA carboxylase. Thus, the signal molecules glucagon, epinephrine, and insulin act in concert on triacylglycerol metabolism and acetyl CoA carboxylase 1 to carefully regulate the utilization and storage of fatty acids.
QUICK QUIZ 2
How are fatty acid synthesis and degradation coordinated?
Malonyl CoA, the substrate for fatty acid synthesis, inhibits carnitine acyl transferase I, thus preventing the transport of fatty acids into mitochondria for degradation. Palmitoyl CoA inhibits acetyl CoA carboxylase, the transport of citrate into the cytoplasm, and glucose 6-
Response to dietLong-