
28.4 Metabolism in Context: Ethanol Alters Energy Metabolism in the Liver
Ethanol has been a part of the human diet for centuries, partly because of its intoxicating effects and partly because alcoholic beverages provided a safe means of hydration when pure water was scarce (Figure 28.11). Indeed, throughout the world, only water and tea are consumed more frequently than beer. However, ethanol consumption in excess can result in a number of health problems, most notably liver damage. What is the biochemical basis of these health problems?

Ethanol cannot be excreted and must be metabolized, primarily by the liver. There are several pathways for the metabolism of ethanol. One pathway consists of two steps. The first step takes place in the cytoplasm:
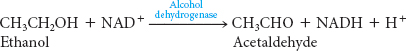
The second step takes place in mitochondria:

Note that ethanol consumption leads to an accumulation of NADH. This high concentration of NADH inhibits gluconeogenesis by preventing the oxidation of lactate to pyruvate. In fact, the high concentration of NADH will cause the reverse reaction to predominate: lactate will accumulate. The consequences may be hypoglycemia (low concentration of blood glucose) and lactic acidosis.
The NADH glut also inhibits fatty acid oxidation. The metabolic purpose of fatty acid oxidation is to generate NADH for ATP generation by oxidative phosphorylation (Chapter 27). However, an alcohol consumer’s NADH needs are met by ethanol metabolism. In fact, the excess NADH signals that conditions are right for fatty acid synthesis. Hence, triacylglycerols accumulate in the liver, leading to a condition known as “fatty liver.”
What are the effects of the other metabolites of ethanol? Liver mitochondria can convert acetate into acetyl CoA in a reaction requiring ATP. The enzyme is the one that normally activates fatty acids—
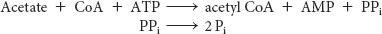
However, further processing of the acetyl CoA by the citric acid cycle is blocked because NADH inhibits two important citric acid cycle regulatory enzymes—