PROBLEMS
Question 28.1
1. Making a fat. Palmitate is a common fatty acid. What is the overall stoichiometry for the synthesis of palmitate from acetyl CoA? ✓ 3
Question 28.2
2. NADH to NADPH. What are the three reactions that allow the conversion of cytoplasmic NADH into NADPH? What enzymes are required? Show the sum of the three reactions. ✓ 3
Question 28.3
3. A pledge to move forward. What is the committed step in fatty acid synthesis, and which enzyme catalyzes the step? ✓ 4
Question 28.4
4. Like sugar ’n’ spice. Match each term (a–
ATP- Malic enzyme Malonyl CoA Acetyl CoA carboxylase 1 Acyl carrier protein β-Ketoacyl synthase Palmitate Eicosanoids Arachidonate AMP- | Catalyzes the committed step in fatty acid synthesis The end product of fatty acid synthase Activated acetyl CoA Molecule on which fatty acids are synthesized Fatty acids containing 20 carbon atoms A precursor of prostaglandins Helps to generate NADPH from NADH Catalyzes the reaction of acetyl CoA and malonyl CoA Inactivates acetyl CoA carboxylase 1 Generates cytoplasmic acetyl CoA |
Question 28.5
5. A supple synthesis. Myristate, a saturated C14 fatty acid, is used as an emollient for cosmetics and topical medicinal preparations. Write a balanced equation for the synthesis of myristate. ✓ 3
Question 28.6
6. The cost of cleanliness. Lauric acid is a 12-
Question 28.7
7. Proper organization. Arrange the following steps in fatty acid synthesis in their proper order. ✓ 3
(a) Dehydration
(b) Condensation
(c) Release of a C16 fatty acid
(d) Reduction of a carbonyl
(e) Formation of malonyl ACP
Question 28.8
8. No access to assets. What would be the effect on fatty acid synthesis of a mutation in ATP-
Question 28.9
9. The truth and nothing but. Indicate whether each of the following statements is true or false. If false, explain.
(a) Biotin is required for fatty acid synthase activity.
(b) The condensation reaction in fatty acid synthesis is powered by the decarboxylation of malonyl CoA.
(c) Fatty acid synthesis does not depend on ATP.
(d) Palmitate is the end product of fatty acid synthesis.
(e) All of the enzyme activities required for fatty acid synthesis in mammals are contained in a single polypeptide chain.
(f) Fatty acid synthase in mammals is active as a monomer.
(g) The fatty acid arachidonate is a precursor for signal molecules.
(h) Acetyl CoA carboxylase 1 is inhibited by citrate.
Question 28.10
10. Odd fat out. Suggest how fatty acids with odd numbers of carbon atoms are synthesized.
Question 28.11
11. Alpha or omega? Only one acetyl CoA molecule is used directly in fatty acid synthesis. Identify the carbon atoms in palmitic acid that were donated by acetyl CoA. ✓ 3
Question 28.12
12. Now you see it, now you don’t. Although HCO3− is required for fatty acid synthesis, its carbon atom does not appear in the product. Explain. ✓ 4
Question 28.13
13. The fizz in fatty acid synthesis. During the initial in vitro studies on fatty acid synthesis, experiments were performed to determine which buffer would result in optimal activity. Bicarbonate buffer proved far superior to phosphate buffer. The researchers could not initially account for this result. From your perspective of many decades later, explain why the result is not surprising.
Question 28.14
14. Tracing carbon atoms. Consider a cell extract that actively synthesizes palmitate. Suppose that a fatty acid synthase in this preparation forms one molecule of palmitate in about 5 minutes. A large amount of malonyl CoA labeled with 14C in each carbon atom of its malonyl unit is suddenly added to this system, and fatty acid synthesis is stopped a minute later by altering the pH. The fatty acids are analyzed for radioactivity. Which carbon atom of the palmitate formed by this system is more radioactive—
Question 28.15
15. Driven by decarboxylation. What is the role of decarboxylation in fatty acid synthesis? Name another key reaction in a metabolic pathway that employs this mechanistic motif.
Question 28.16
16. An unaccepting mutant. The serine residue in acetyl CoA carboxylase 1 that is the target of the AMP-
Question 28.17
17. All for one, one for all. What is a potential disadvantage of having many catalytic sites together on one very long polypeptide chain?
Question 28.18
18. Six of one, half a dozen of the other. People who consume little fat but excess carbohydrates can still become obese. How is this result possible?
Question 28.19
19. No traffic allowed. Both fatty acid synthesis and fatty acid degradation are regulated, at least in part, by controlling the movement of molecules into and out of mitochondria. Describe two such examples. ✓ 4
Chapter Integration Problems
Question 28.20
20. A tight embrace. Avidin, a glycoprotein protein found in eggs, has a high affinity for biotin. Avidin can bind biotin and prevent its use by the body. How might a diet rich in raw eggs affect fatty acid synthesis? What will be the effect on fatty acid synthesis of a diet rich in cooked eggs? Explain.
Question 28.21
21. All about communication. Why is citrate an appropriate inhibitor of phosphofructokinase?
Question 28.22
22. Counterpoint. Compare and contrast fatty acid oxidation and synthesis with respect to (a) site of the process; (b) acyl carrier; (c) reductants and oxidants; (d) stereo-
Question 28.23
23. Familiars. One of the reactions critical for the generation of cytoplasmic NADPH from cytoplasmic NADH is also important in gluconeogenesis. What is the reaction and what is the immediate fate of the product of the reaction in gluconeogenesis?
Question 28.24
24. A foot in both camps. What role does acetyl CoA carboxylase play in the regulation of fatty acid degradation? ✓ 4
Data Interpretation Problem
Question 28.25
25. Coming together. The graph below shows the response of phosphorylated acetyl CoA carboxylase 1 to varying amounts of citrate. Explain this effect in light of the allosteric effects that citrate has on the enzyme. Predict the effects of increasing concentrations of palmitoyl CoA. ✓ 4
Question 28.26
26. Nip it in the bud. Soraphen A is a natural antifungal agent isolated from a myxobacterium. Soraphen A is also a potent inhibitor of acetyl CoA carboxylase 1, the regulatory enzyme that initiates fatty acid synthesis. As discussed earlier, cancer cells must produce large amounts of fatty acids to generate phospholipids for membrane synthesis. Thus, acetyl CoA carboxylase 1 might be a target for anticancer drugs. Below are the results of experiments testing this hypothesis on a cancer line.
Figure A shows the effect of various amounts of soraphen A on fatty acid synthesis as measured by the incorporation of radioactive acetate (14C) into fatty acids.
(a) How did soraphen A alter fatty acids synthesis? How was synthesis affected by increasing concentrations of soraphen A?
Figure B shows the results obtained when fatty acid oxidation was measured, as the release of radioactive CO2 from added radioactive (14C) palmitate, in the presence of soraphen A.
(b) How did soraphen A alter fatty acid oxidation?
(c) Explain the results obtained in B in light of the fact that soraphen A inhibits acetyl CoA carboxylase 1.
More experiments were undertaken to assess whether carboxylase inhibition did indeed prevent phospholipid synthesis. Again, cells were grown in the presence of radioactive acetate, and phospholipids were subsequently isolated and the amount of 14C incorporated into the phospholipid was determined. The effect of soraphen A on phospholipid synthesis is shown in Figure C.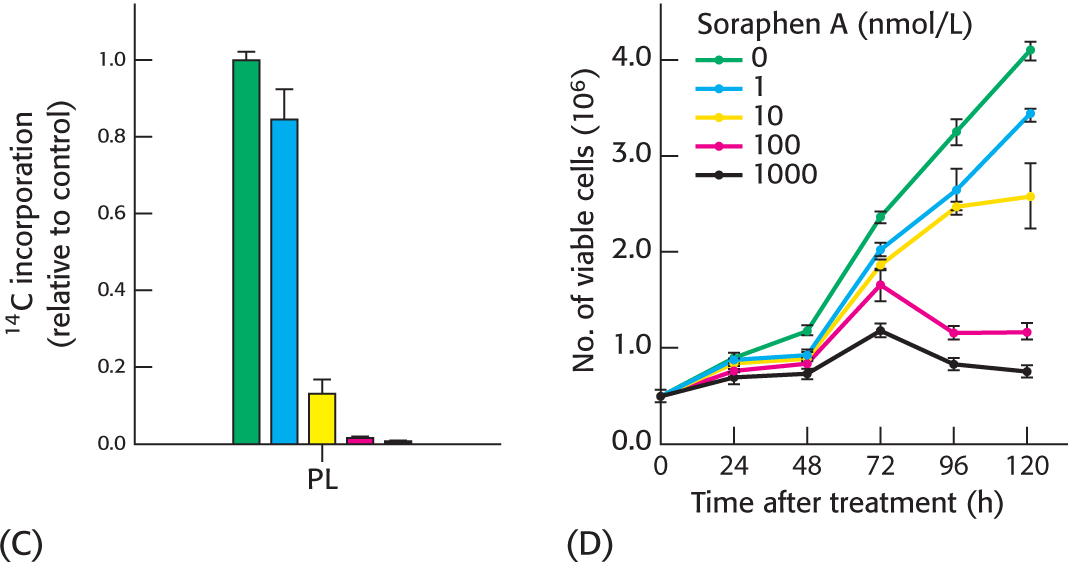
(d) Did the inhibition of carboxylase 1 by the drug alter phospholipid synthesis?
(e) How might phospholipid synthesis effect cell viability?
Finally, as shown in Figure D, experiments were performed to determine if the drug inhibits cancer growth in vitro by determining the number of cancer cells surviving upon exposure to soraphen A.
(f) How did soraphen A effect cancer cell viability?
[Data for graphs A–
Challenge Problems
Question 28.27
27. Labels. Suppose that you had an in vitro fatty-
The ratio of 3H/14C is 3. What would the 3H/14C ratio be after the synthesis of palmitic acid (C16) with the use of the radioactive acetyl CoA?
Question 28.28
28. If a little is good, a lot is not better. Ethanol can be converted into acetate in the liver by alcohol dehydrogenase and aldehyde dehydrogenases: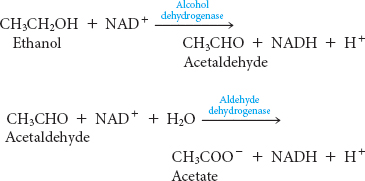
These reactions alter the NAD+/NADH ratio in the liver. What effect will this alteration have on glycolysis, gluconeogenesis, fatty acid metabolism, and the citric acid cycle? ✓ 4
Selected Readings for this chapter can be found online at www.whfreeman.com/