29.2 Cholesterol Is Synthesized from Acetyl Coenzyme A in Three Stages
DID YOU KNOW?
“Cholesterol is the most highly decorated small molecule in biology. Thirteen Nobel Prizes have been awarded to scientists who devoted major parts of their careers to cholesterol. Ever since it was isolated from gallstones in 1784, cholesterol has exerted an almost hypnotic fascination for scientists from the most diverse areas of science and medicine…. Cholesterol is a Janus-
—Michael Brown and Joseph Goldstein, on the occasion of their receipt of the Nobel Prize for elucidating the control of blood levels of cholesterol. Nobel Lectures (1985); © The Nobel Foundation, 1985
We now turn our attention to the synthesis of a different kind of lipid, which lacks the long hydrocarbon chains characteristic of triacylglycerols and membrane lipids—
Cholesterol is synthesized in the liver and, to a lesser extent, in other tissues. The rate of its synthesis is highly responsive to the cellular level of cholesterol. All 27 carbon atoms of cholesterol are derived from acetyl CoA in a three-
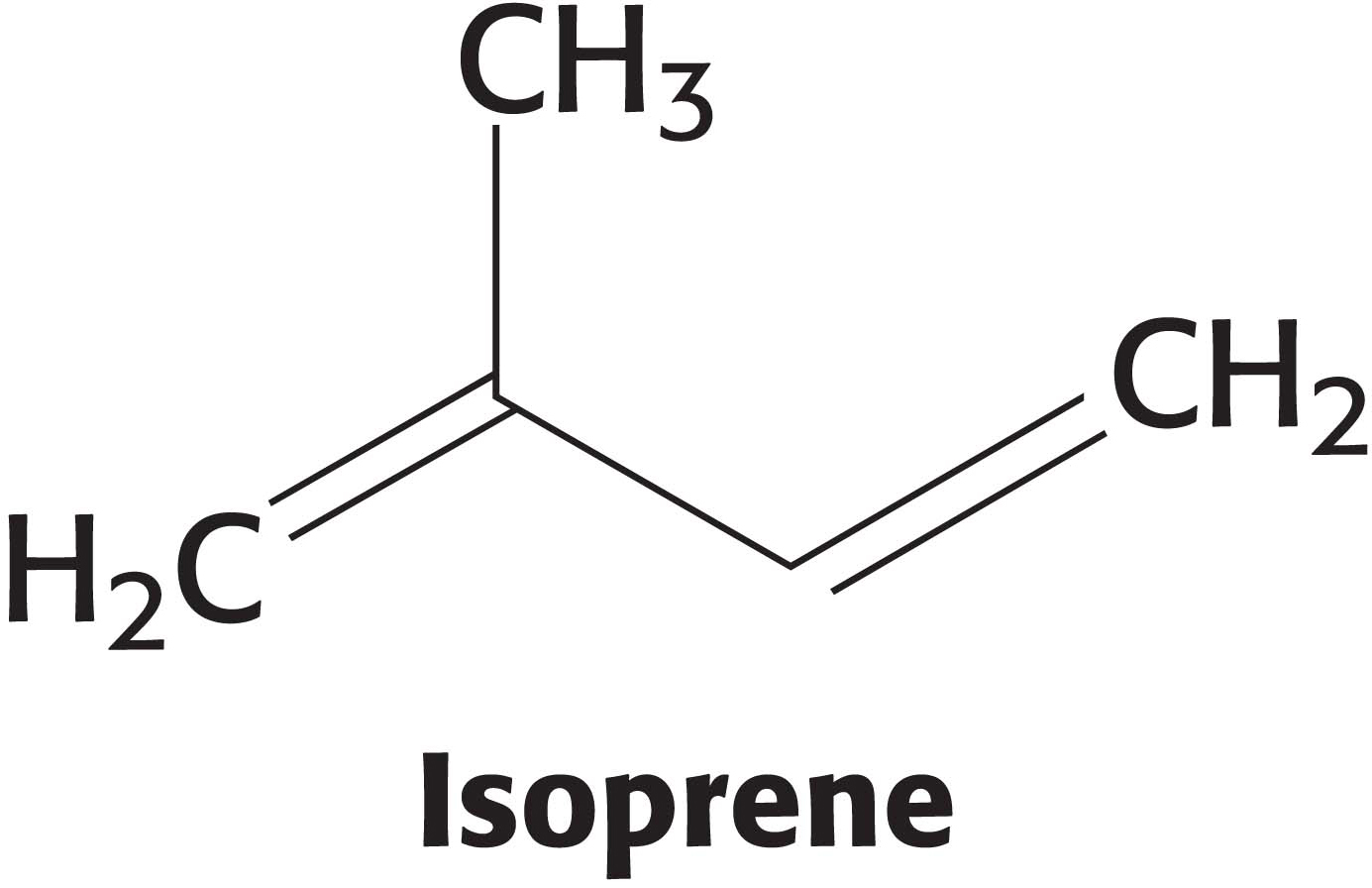
Stage one is the synthesis of isopentenyl pyrophosphate, an activated isoprene unit that is the key building block of cholesterol.
Stage two is the condensation of six molecules of isopentenyl pyrophosphate to form squalene.
In stage three, squalene cyclizes and the tetracyclic product is subsequently converted into cholesterol.
The first stage takes place in the cytoplasm, and the second two in the lumen of the endoplasmic reticulum.
The Synthesis of Mevalonate Initiates the Synthesis of Cholesterol
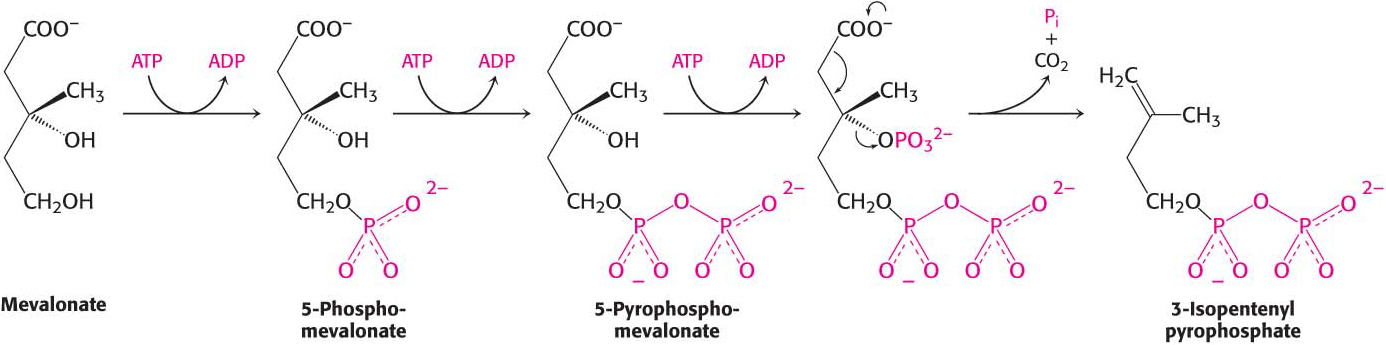
The first stage in the synthesis of cholesterol is the formation of isopentenyl pyrophosphate from acetyl CoA. This set of reactions starts with the formation of 3-
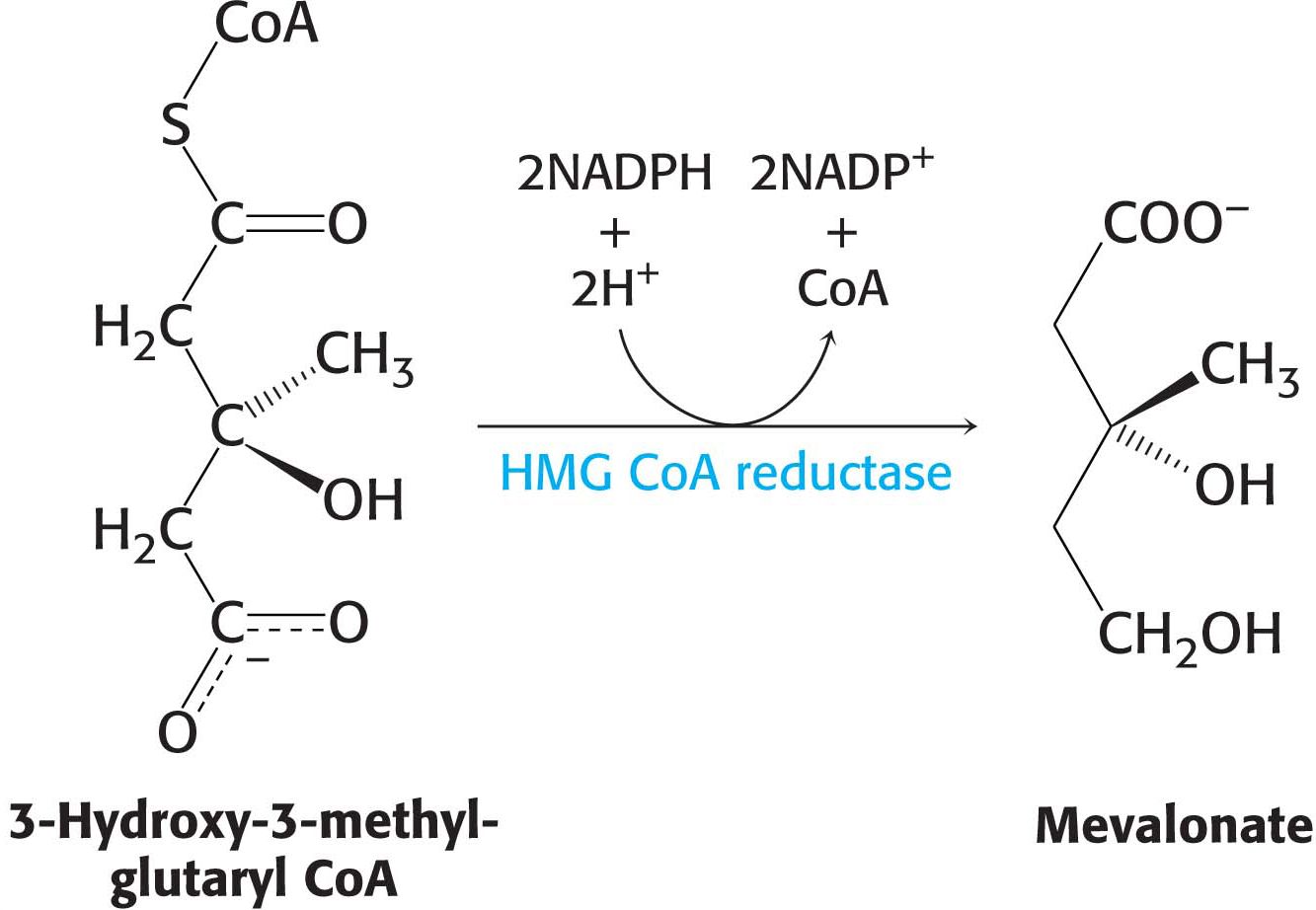
The synthesis of mevalonate is the committed step in cholesterol formation. The enzyme catalyzing this irreversible step, 3-
Mevalonate is converted into 3-
Squalene (C30) Is Synthesized from Six Molecules of Isopentenyl Pyrophosphate (C5)
The next major precursor on the path to cholesterol is squalene, which is synthesized from isopentenyl pyrophosphate by the following reaction sequence:

Before the condensation reactions take place, isopentenyl pyrophosphate isomerizes to dimethylallyl pyrophosphate:
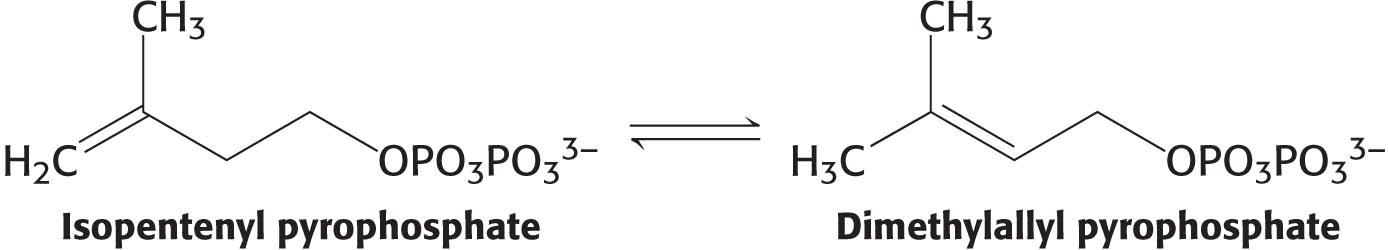
The two isomer C5 units (one of each) condense to begin the formation of squalene. The reactions leading from the six C5 units to squalene, a C30 isoprenoid, are summarized in (Figure 29.9).
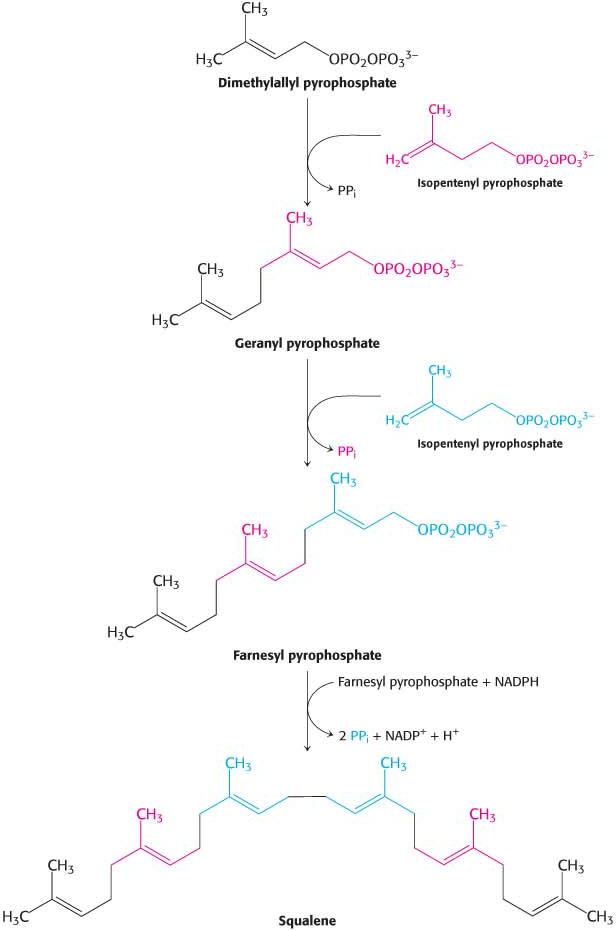
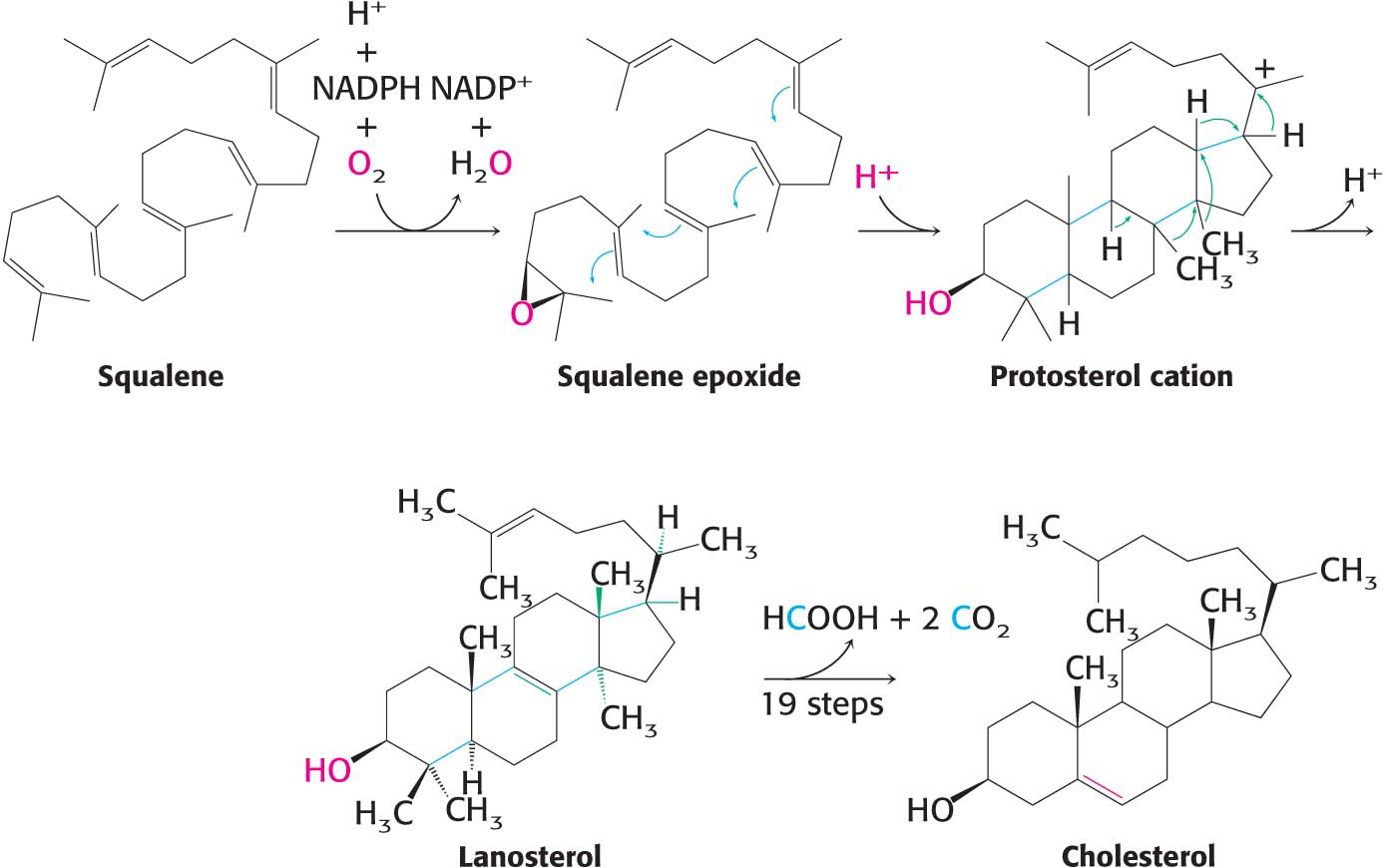
Squalene Cyclizes to Form Cholesterol
DID YOU KNOW?
An epoxide is a three-
In the final stage of cholesterol biosynthesis, squalene cyclizes to form a ringlike structure (Figure 29.10). Squalene is first activated by conversion into squalene epoxide (2,3-