
29.3 The Regulation of Cholesterol Synthesis Takes Place at Several Levels
✓ 6 List the regulatory steps in the control of cholesterol synthesis.
Cholesterol can be obtained from the diet or it can be synthesized de novo. An adult on a low-
The rate of synthesis of HMG-
CoA reductase mRNA is controlled by the sterol regulatory element binding protein (SREBP). This transcription factor binds to a short DNA sequence called the sterol regulatory element (SRE) on the 5′ side of the reductase gene. It binds to the SRE when cholesterol levels are low and enhances transcription. In its inactive state, the SREBP resides in the endoplasmic reticulum membrane, where it is associated with the SREBP cleavage activating protein (SCAP), an integral membrane protein. SCAP is the cholesterol sensor. When cholesterol levels fall, SCAP escorts SREBP in small membrane vesicles to the Golgi complex, where it is released from the membrane by two specific proteolytic cleavages (Figure 29.12). The released protein migrates to the nucleus and binds the SRE of the HMG-CoA reductase gene, as well as several other genes in the cholesterol biosynthetic pathway, to enhance transcription. When cholesterol levels rise, the proteolytic release of the SREBP is blocked, and the SREBP in the nucleus is rapidly degraded. These two events halt the transcription of genes of the cholesterol biosynthetic pathways. 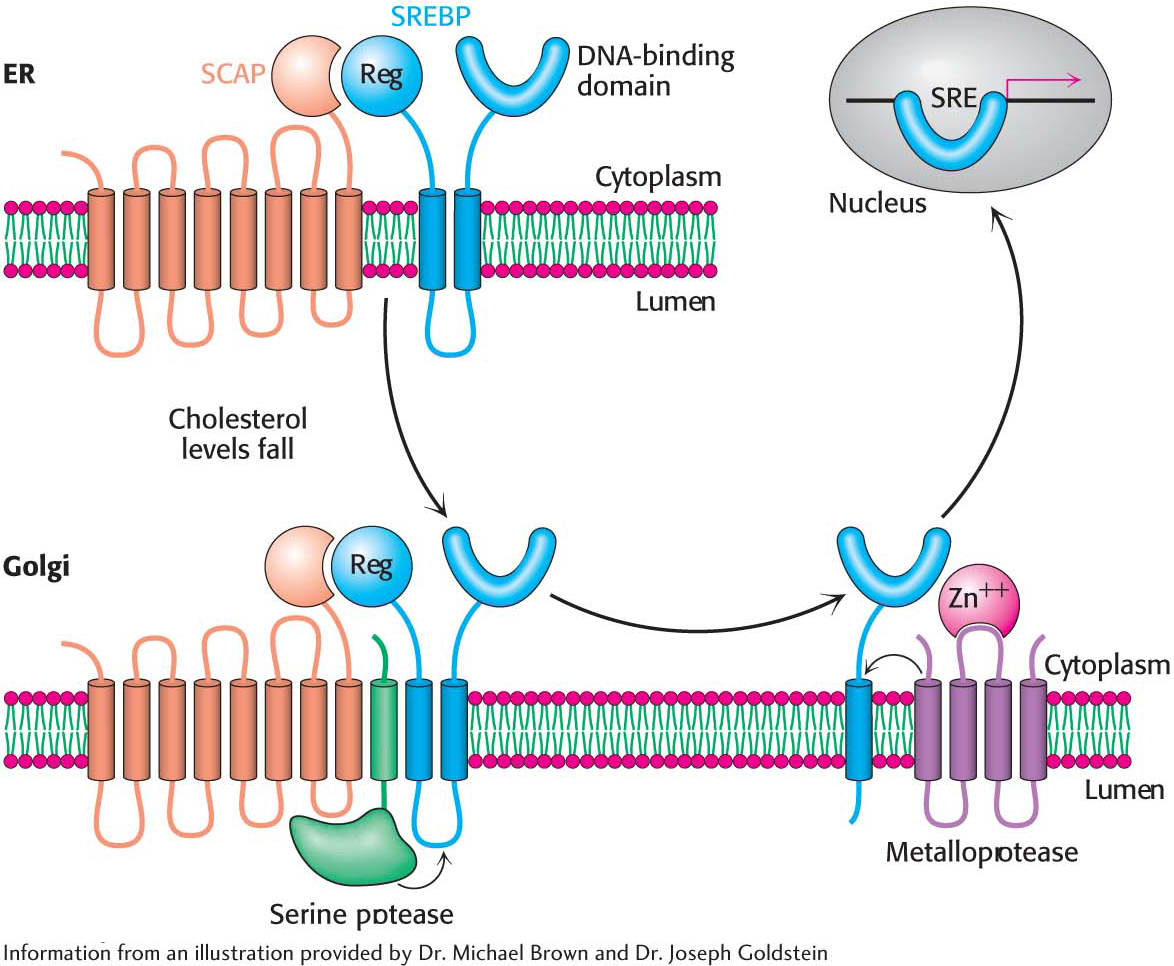 Figure 29.12 The SREBP pathway. SREBP resides in the endoplasmic reticulum, where it is bound to SCAP by its regulatory (Reg) domain. When cholesterol levels fall, SCAP and SREBP move to the Golgi complex, where SREBP undergoes successive proteolytic cleavages by a serine protease and a metalloprotease. The released DNA-
Figure 29.12 The SREBP pathway. SREBP resides in the endoplasmic reticulum, where it is bound to SCAP by its regulatory (Reg) domain. When cholesterol levels fall, SCAP and SREBP move to the Golgi complex, where SREBP undergoes successive proteolytic cleavages by a serine protease and a metalloprotease. The released DNA-binding domain moves to the nucleus to alter gene expression. Page 533What is the molecular mechanism that retains SCAP-
SREBP in the endoplasmic reticulum when cholesterol is present but allows movement to the Golgi complex when the cholesterol concentration is low? When cholesterol is low, SCAP binds to vesicular proteins that facilitate the transport of SCAPSREBP to the Golgi apparatus. When cholesterol is present, SCAP binds cholesterol, which causes a structural change in SCAP so that it binds to Insig (insulin- induced gene), another endoplasmic reticulum protein. Insig is the anchor that retains SCAP and thus SREBP in the endoplasmic reticulum in the presence of cholesterol. The rate of translation of HMG-
CoA reductase mRNA is inhibited by nonsterol metabolites derived from mevalonate as well as by dietary cholesterol.The degradation of HMG-
CoA reductase is stringently controlled . The enzyme has two domains: its cytoplasmic domain carries out catalysis, and its membrane domain senses signals that lead to its degradation. The membrane domain may undergo structural changes in response to increasing concentrations of sterols such as cholesterol that make the enzyme more susceptible to proteolysis. A combination of these three regulatory devices can alter the amount of enzyme in the cell more than 200-fold. Page 534- ?
QUICK QUIZ 2
Outline the mechanisms of regulation of cholesterol biosynthesis.
The amount of reductase and its activity control the regulation of cholesterol biosynthesis. Transcriptional control is mediated by SREBP. Translation of the reductase mRNA also is controlled. The reductase itself may undergo regulated proteolysis. Finally, the activity of the reductase is inhibited by phosphorylation by AMP-
activated kinase when ATP levels are low. Phosphorylation decreases the activity of HMG-
CoA reductase . This enzyme, like acetyl CoA carboxylase (which catalyzes the committed step in fatty acid synthesis), is switched off by an AMP-activated protein kinase. Thus, cholesterol synthesis ceases when the ATP level is low.
We will return to the control of cholesterol in a clinical context after we consider a related topic—