
2.2 Biochemical Interactions Take Place in an Aqueous Solution
✓ 4 Describe the chemical properties of water and explain how water affects biochemical interactions.
Water is the solvent of life. Human beings are 65% water, tomatoes are 90% water, and a typical cell is about 70% water. Indeed, most organisms are mostly water, be they bacteria, cacti, whales, or elephants. Many of the organic molecules requiredfor the biochemistry of living systems dissolve in water. In essence, water rendersmolecules mobile and permits Brownian-
Water is a simple molecule, composed of two hydrogen atoms linked by covalent bonds to a single atom of oxygen. The important properties of water are due to the fact that oxygen is an electronegative atom. That is, although the bonds joining the hydrogen atoms to the oxygen atom are covalent, the electrons of the bond spend more time near the oxygen atom. Because the charge distribution is not uniform, the water molecule is said to be polar. The oxygen atom is slightly negatively charged (designated δ−), and the hydrogen atoms are correspondingly slightly positively charged (δ+).
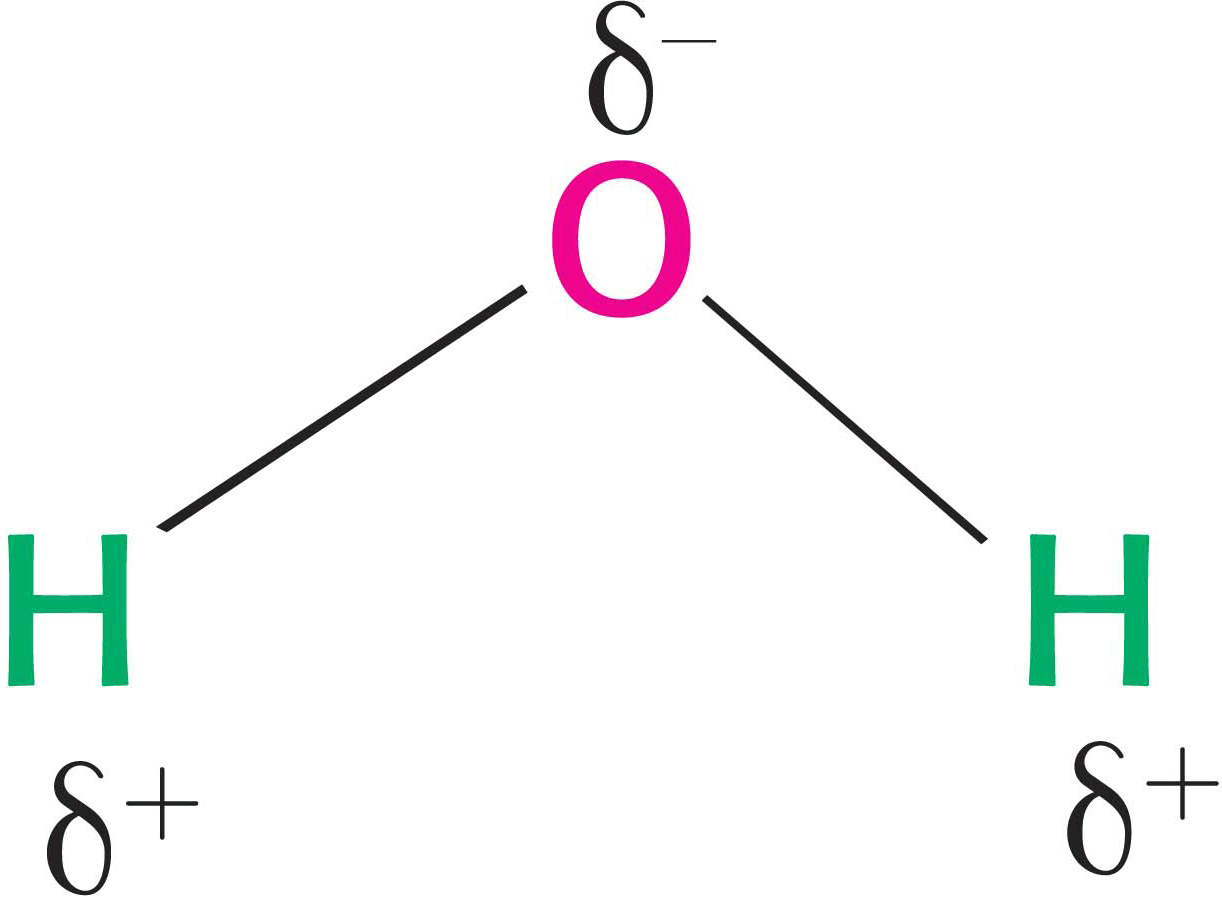
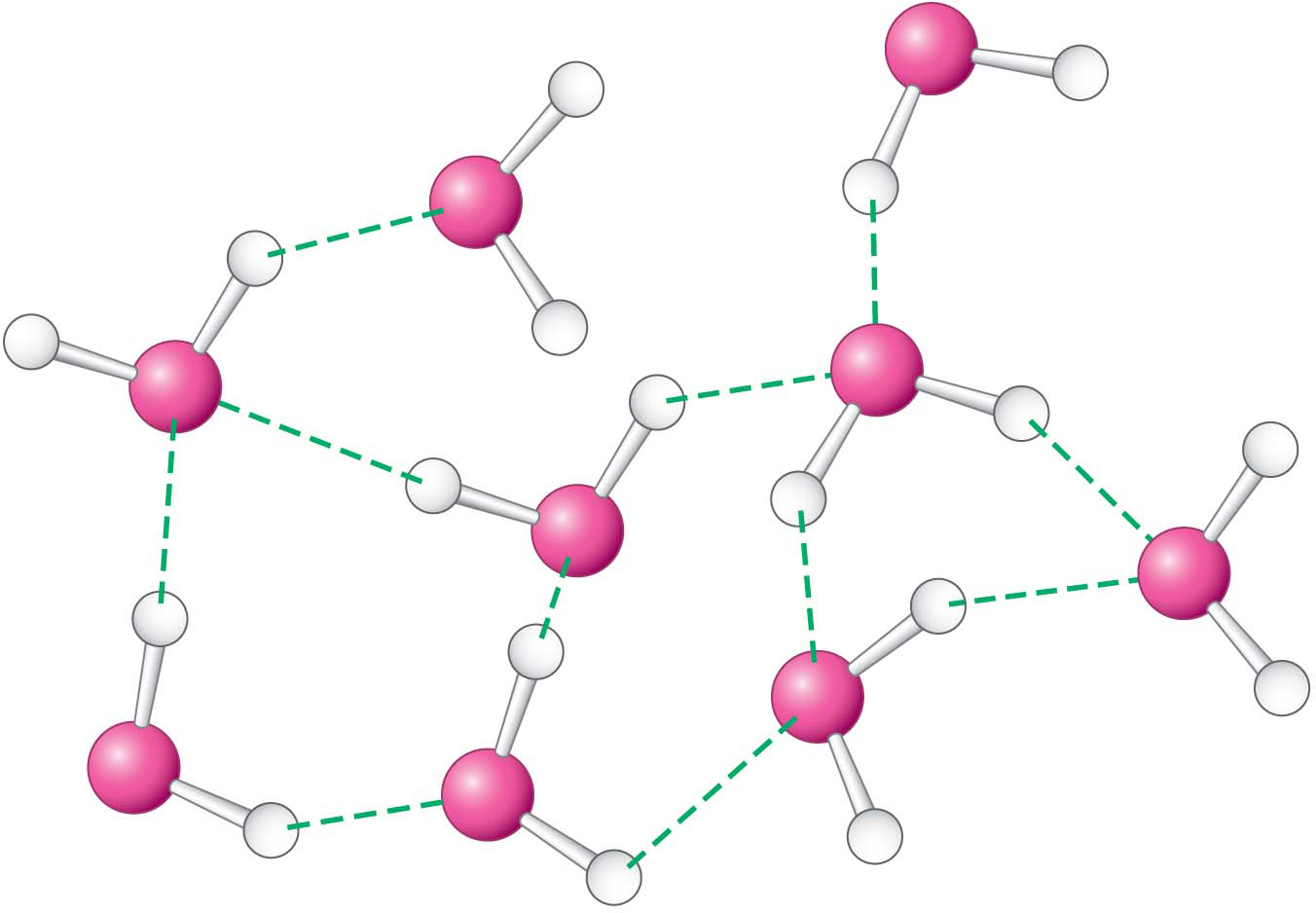
This polarity has important chemical ramifications. The partially positively charged hydrogen atoms of one molecule of water can interact with the partially negatively charged oxygen atoms of another molecule of water. This interaction is called a hydrogen bond (Figure 2.1). As we will see, hydrogen bonds are not unique to water molecules and in fact are common weak bonds in biomolecules. Liquid water has a partly ordered structure in which hydrogen-

The giant trees of the redwood forests—
Although water’s ability to dissolve many biochemicals is vitally important, the fact that water cannot dissolve certain compounds is equally important. A certain class of molecules termed nonpolar, or hydrophobic, cannot dissolve in water. These molecules, in the presence of water, behave exactly as the oil in an oil-