2.4 Hydrophobic Molecules Cluster Together
The existence of life on Earth depends critically on the capacity of water to dissolve polar or charged molecules. However, not all biomolecules are polar or ionic. Such molecules are called nonpolar or hydrophobic molecules because these molecules simply cannot interact with water. Oil-
To understand how this organization takes place, we need to refer to the Second Law of Thermodynamics:
The total entropy of a system and its surroundings always increases in a spontaneous process.
Entropy is a measure of randomness. The system may be a chemical reaction, a cell, or a bottle of salad dressing. Consider the introduction of a single nonpolar molecule, such as benzene, into some water (Figure 2.9).
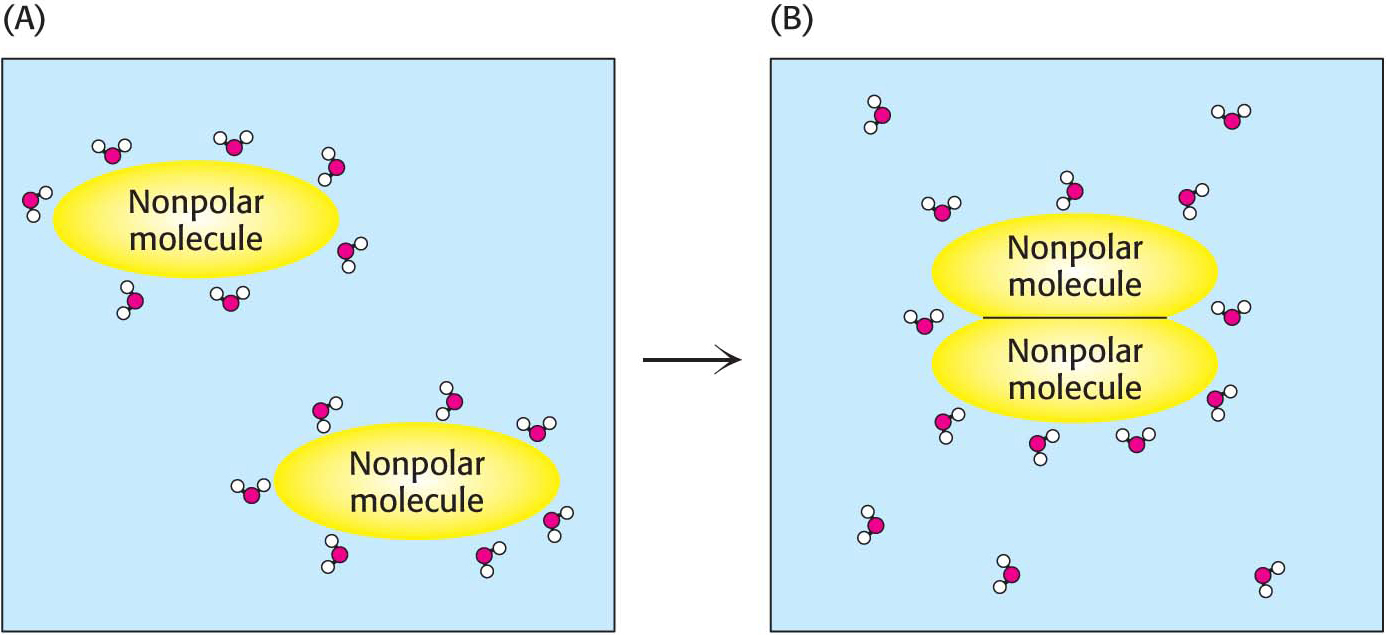
A cavity in the water is created because the benzene has no chemical means of interacting with the water molecule. The cavity temporarily disrupts some hydrogen bonds between water molecules. The displaced water molecules then reorient themselves to form the maximum number of new hydrogen bonds. However, there are many fewer ways of forming hydrogen bonds around the benzene molecule than there are in pure water. The water molecules around the benzene molecule are much more ordered than elsewhere in the solution. The introduction of the nonpolar molecule into water has resulted in a decrease in the entropy of water. Now consider the arrangement of two benzene molecules in water. They do not reside in separate small cavities (Figure 2.9A); instead, they coalesce into a single larger one (Figure 2.9B). They become organized. The energetic basis for the formation of this order is that the association of the benzene molecules releases some of the ordered water molecules around the separated benzenes, increasing the entropy of the system. Nonpolar solute molecules are driven together in water not primarily because they have a high affinity for each other but because, when they do associate, they release water molecules. This entropy-
Membrane Formation Is Powered by the Hydrophobic Effect
The biological significance of the hydrophobic effect is more apparent when we consider molecules more complex than benzene, such as a phospholipid (Chapter 11). Recall that the structure of the phospholipid reveals two distinct chemical properties (Figure 1.4). The top of the molecule, called the head group, is hydrophilic, consisting of polar and charged species. However, the remainder of the molecule, consisting of two large hydrophobic chains, cannot interact with water. Such a molecule, with two distinct chemical personalities, is called an amphipathic or amphiphilic molecule. When exposed to water, the molecules orient themselves such that the hydrophilic head groups interact with the aqueous medium, whereas the hydrophobic tails are sequestered away from the water and interact only with one another. Under the right conditions, they can form membranes. The lipids form a contiguous, closed bilayer, with two hydrophilic outsides and a hydrophobic interior, which is stabilized by van der Waals interactions between the hydrophobic tails.
Membranes define the inside and outside of the cell, as well as separating the components of eukaryotic cells into distinct biochemical compartments. Paradoxically, order has been introduced by an increase in the randomness of water. We will return to the topic of membranes many times in this book, because membranes are vital to many aspects of energy and information transformation.
Protein Folding Is Powered by the Hydrophobic Effect
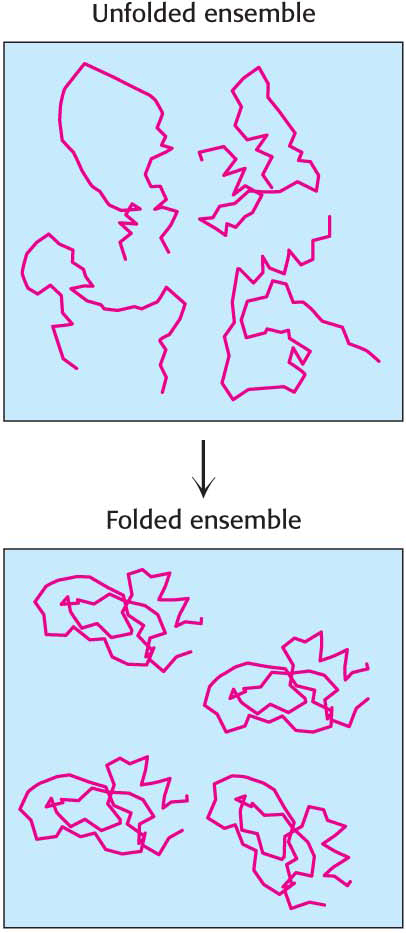
Proteins, which we will consider in Chapters 3 and 4, are the true workhorses of biochemistry, playing prominent roles in all aspects of energy and information manipulation. Proteins play these roles because they are capable of forming complex three-
QUICK QUIZ 2
Explain how the following statement applies to biochemistry: Order can be generated by an increase in randomness.
The statement essentially describes the hydrophobic effect. Specific complicated biochemical structures can form, powered by the increase in entropy that results when hydrophobic groups are removed from aqueous solution.
How can we reconcile the apparent contradiction that proteins spontaneously assume an ordered structure, and yet entropy increases? We can again call on the hydrophobic effect to introduce order. Some of the amino acids that make up proteins have nonpolar groups. These nonpolar amino acids have a strong tendency to associate with one another in the interior of the folded protein. The increased entropy of water resulting from the interaction of these hydrophobic amino acids helps to compensate for the entropy losses inherent in the folding process. Thus, the same thermodynamic principles that permit the formation of membranes facilitate the formation of the intricate three-
Although the hydrophobic effect powers the folding of proteins, many weak bonds, including hydrogen bonds and van der Waals interactions, are formed in the protein-
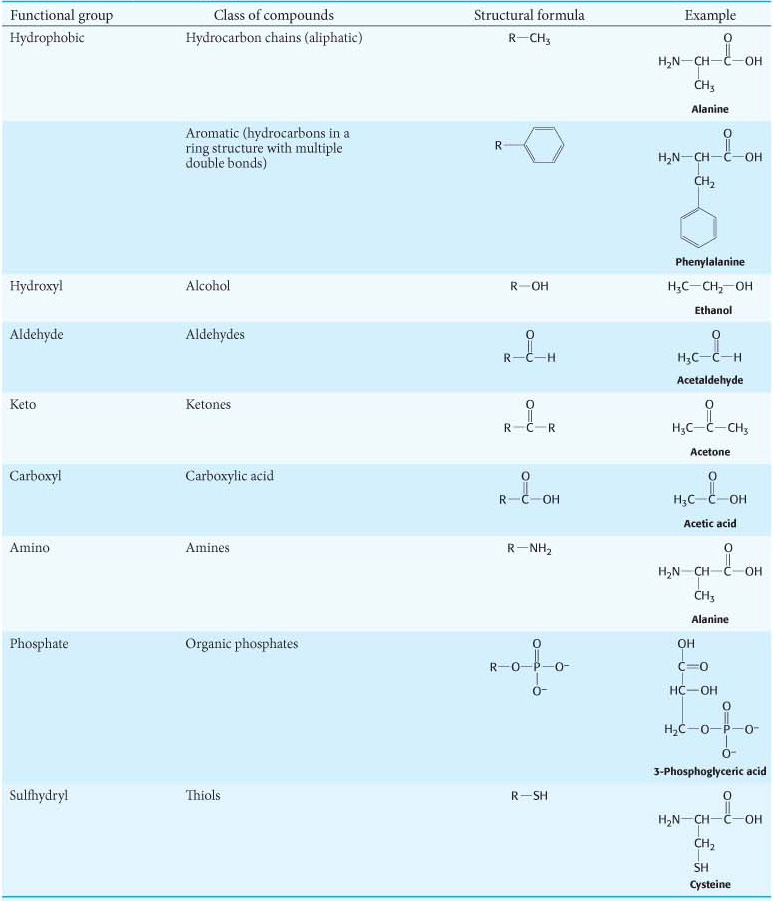
Functional Groups Have Specific Chemical Properties
As we progress in our study of biochemistry, we will see a dizzying number of biomolecules. However, there are generalizations that make dealing with the large number of molecules easier. All biomolecules interact with one another and their environment by using the three types of reversible interactions and the hydrophobic effect, as heretofore discussed. As stated in Chapter 1, there are only four major classes of biomolecules. We can make the chemical basis of biochemistry even more manageable by noting that a limited number of groups of atoms with distinct chemical properties, called functional groups, are found in all biomolecules, including the four classes considered in Chapter 1 (Table 2.1). Each of the eight common functional groups listed in Table 2.1 confers similar chemical properties on the molecules of which it is a component. These groups are called functional groups because the chemical properties conferred are necessary for the biochemical function of the molecules. Note that all of these groups have hydrogen-