PROBLEMS
Question 2.1
1. A random walk. Define Brownian motion.
Question 2.2
2. Inequality. Water is said to be polar but uncharged. How is it possible? ✓ 4
Question 2.3
3. United we stand. Why are weak bonds important in biochemistry? ✓ 4
Question 2.4
4. Bond types. What are the common types of weak bonds important in biochemistry? How does water affect these bonds? ✓ 4
Question 2.5
5. Temperature and bonds. In liquid water, each molecule is hydrogen bonded to approximately 3.4 molecules of water. What effect would freezing water have on the number of hydrogen bonds? Heating water?
Question 2.6
6. Context matters. What would be the effect of an organic solvent on electrostatic interactions? ✓ 5
Question 2.7
7. Some atoms don’t share well. What is an electronegative atom, and why are such atoms important in biochemistry? ✓ 5
Question 2.8
8. Oil and vinegar. Define the hydrophobic effect. ✓ 5
Question 2.9
9. Laws are important. How does the Second Law of Thermodynamics allow for the formation of biochemical order?
Question 2.10
10. Fourteen once. If an aqueous solution has a hydrogen ion concentration of 10−5 M, what is the concentration of hydroxyl ion? ✓ 6
Question 2.11
11. Fourteen twice. If an aqueous solution has a hydroxyl ion concentration of 10−2 M, what is the concentration of hydrogen ion? ✓ 6
Question 2.12
12. Acid–base chemistry. Using the Henderson–
Question 2.13
13. Acid strength. What is the relation between the pKa of an acid and the strength of the acid? ✓ 6
Question 2.14
14. Warts beware. The pKa of acetic acid is 4.76 and the pKa of trichloroacetic acid, which is used to remove warts, is 0.7. Calculate the dissociation constant of each acid. Which is the stronger acid? ✓6
Question 2.15
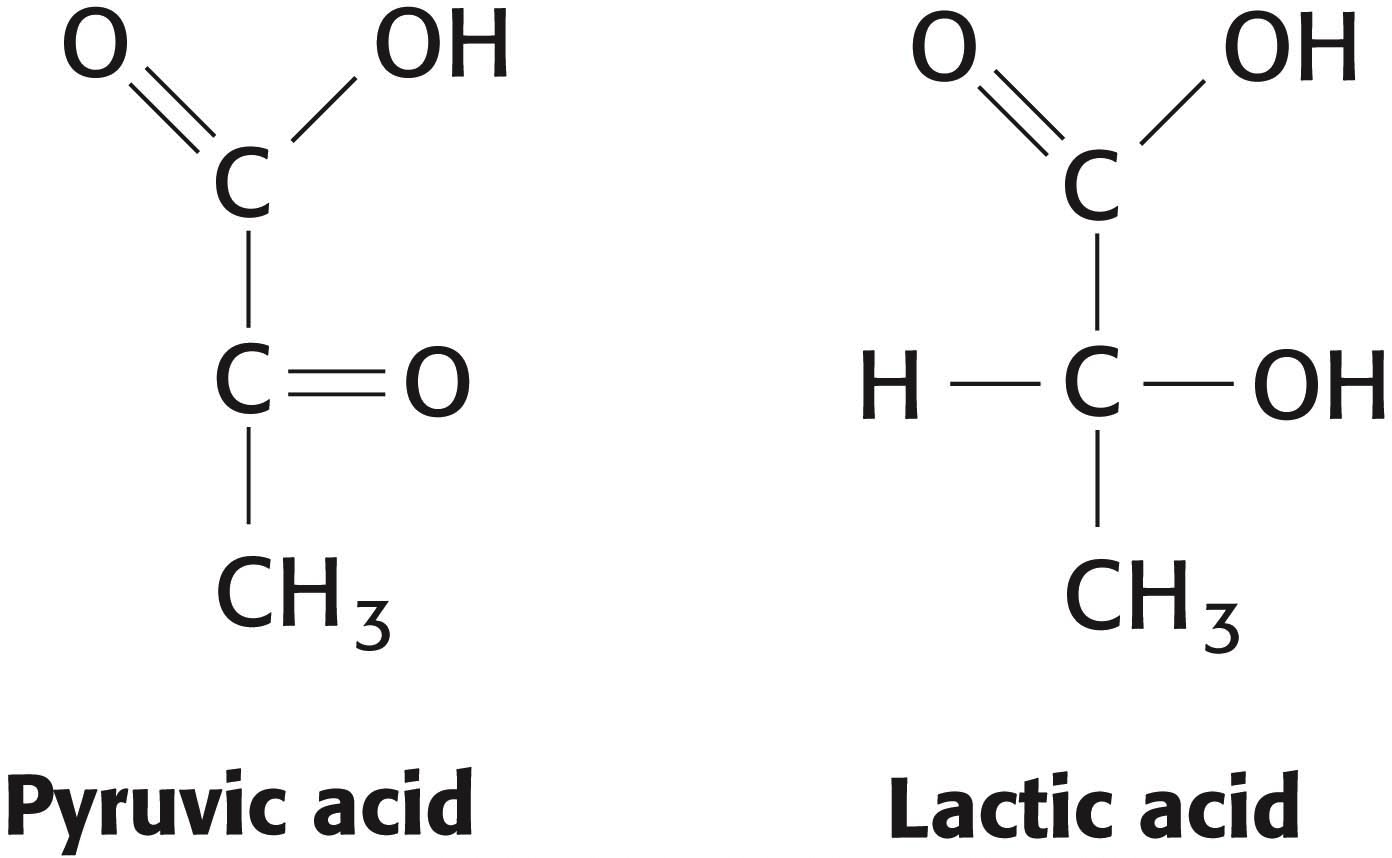
15. Acids from the health food store? Many important biochemicals are organic acids, such as pyruvic acid (pKa = 2.50) and lactic acid (pKa = 3.86). The conjugate bases are pyruvate and lactate, respectively. Determine, for each acid, which form—
Challenge Problems
Question 2.16
16. Find the pKa. For an acid HA, the concentrations of HA and A− are 0.075 and 0.025, respectively, at pH 6.0. What is the pKa value for HA? ✓ 6
Question 2.17
17. pH indicator. A dye that is an acid and that appears as different colors in its protonated and deprotonated forms can be used as a pH indicator. Suppose that you have a 0.001 M solution of a dye with a pKa of 7.2. From the color, the concentration of the protonated form is found to be 0.0002 M. Assume that the remainder of the dye is in the deprotonated form. What is the pH of the solution? ✓ 6
Question 2.18
18. What’s the ratio? An acid with a pKa of 8.0 is present in a solution with a pH of 6.0. What is the ratio of the protonated to the deprotonated form of the acid? ✓ 6
Question 2.19
19. Buffer capacity. Two solutions of sodium acetate are prepared, one having a concentration of 0.1 M and the other having a concentration of 0.01 M. Calculate the pH values when the following concentrations of HCl have been added to each of these solutions: 0.0025 M, 0.005 M, 0.01 M, and 0.05 M. ✓ 6
Question 2.20
20. Another ratio. Calculate the concentration of acetic acid and acetate ion in a 0.2 M acetate buffer at pH 5. The pKa of acetic acid is 4.76. ✓ 6
Question 2.21
21. Buffers STAT! You are working in a high-
Question 2.22
22. Blood pH. Following a bout of intense exercise, the pH of the exerciser’s blood was found to be 7.1. If the HCO3− concentration is 8 mM, and the pKa for HCO3− is 6.1, what is the concentration of CO2 in the blood? ✓ 6
Question 2.23
23. Metabolic acidosis. As we will see later in the course, pathological conditions arise where the blood pH falls because of excess acid production, a condition called metabolic acidosis. Excess protons in the blood decrease the amount of HCO3− and thus reduce the buffering capacity of blood. A rapid drop in pH could lead to death. Normal values for blood are: pH = 7.4, [HCO3−] = 24.0 mM, [CO2] = 1.10 mM. ✓ 6
(a) If a patient has a blood pH = 7.03 and [CO2] = 1.1 mM, what is the [HCO3−] in the patient’s blood? The pKa of HCO3− = 6.1.
(b) Suggest a possible treatment for metabolic acidosis.
(c) Why might the suggestion for part (b) be of benefit to middle-