
30.2 Ammonium Ion Is Converted into Urea in Most Terrestrial Vertebrates
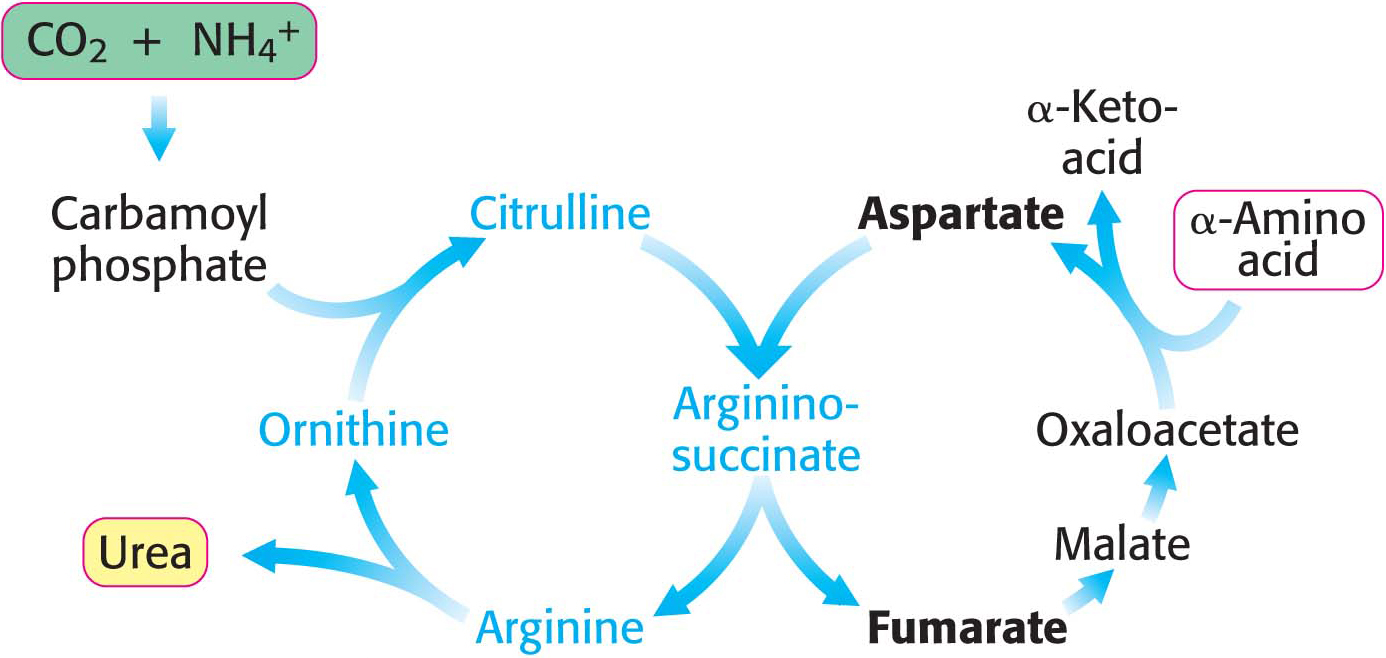
Some of the NH4+ formed in the breakdown of amino acids is consumed in the biosynthesis of nitrogen compounds. In most terrestrial vertebrates, the excess NH4+ is converted into urea by the urea cycle and then excreted (Figure 30.2). Such organisms are referred to as ureotelic. One of the nitrogen atoms of the urea is transferred from an amino acid, aspartate. The other nitrogen atom is derived directly from free NH4+, and the carbon atom comes from HCO3– (derived from the hydration of CO2).
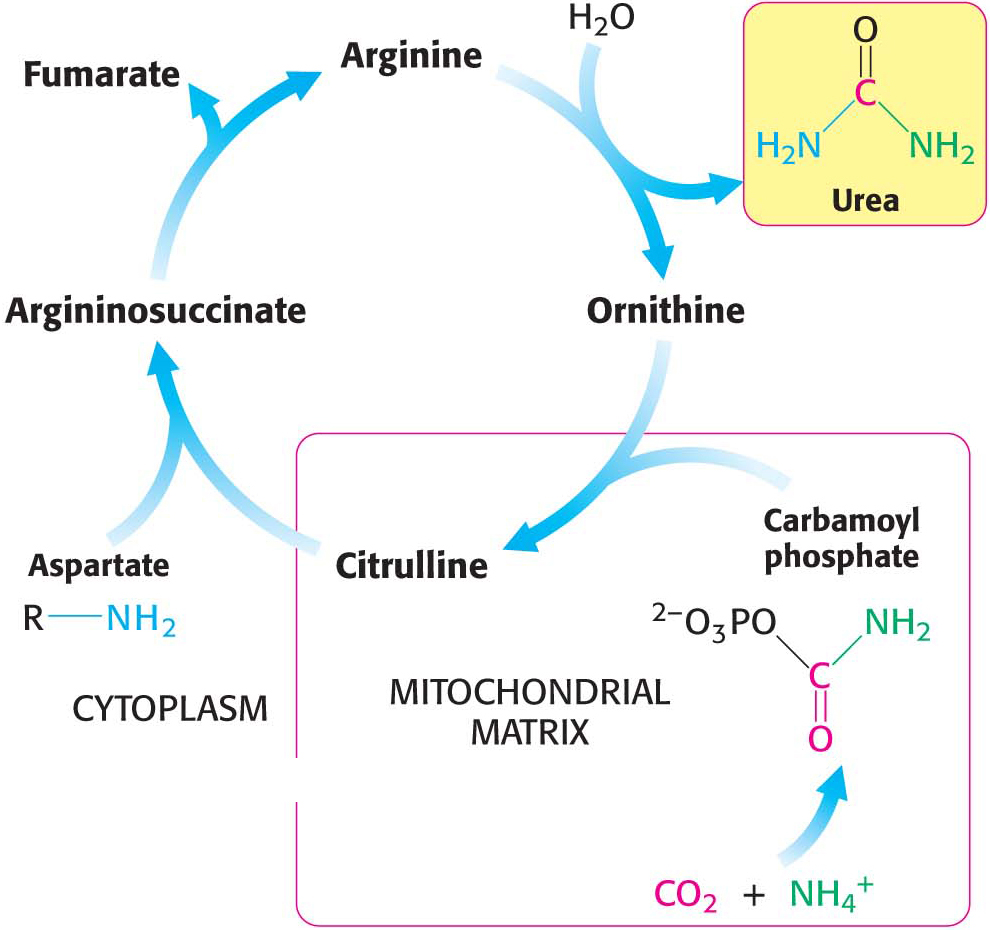
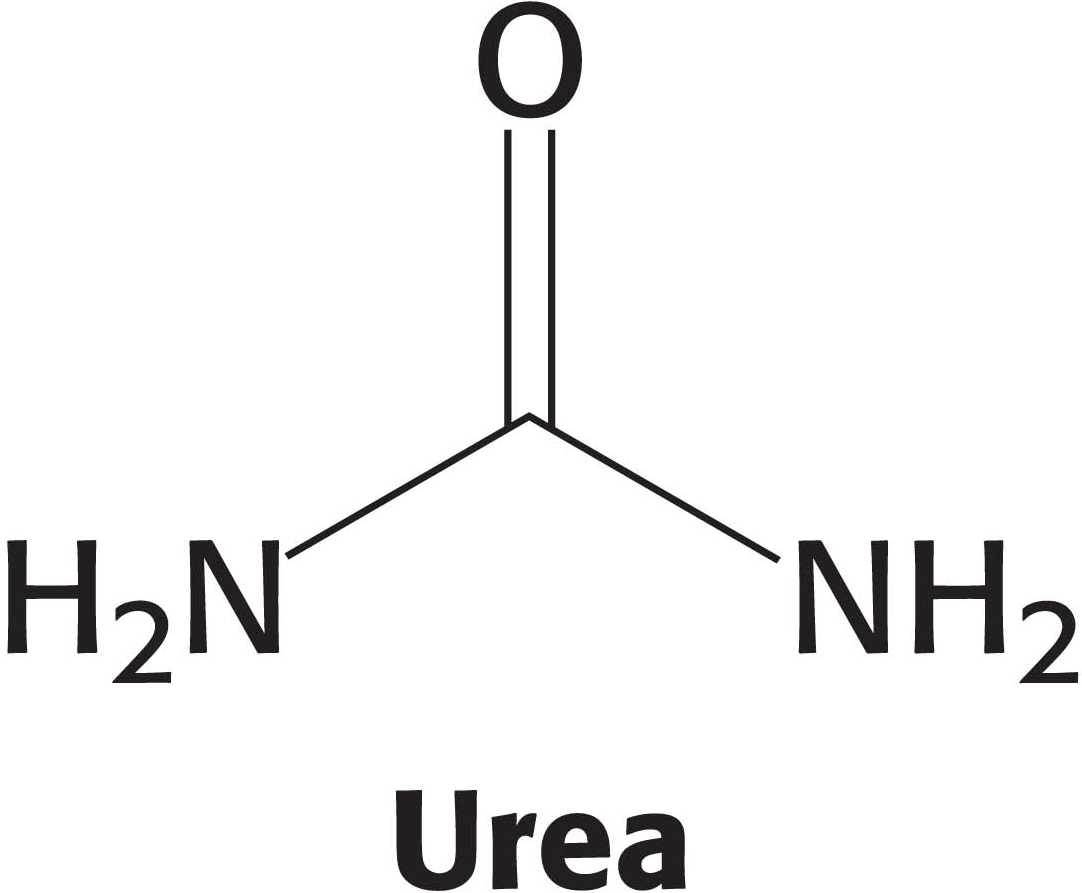
The urea cycle begins in mitochondria with the coupling of free NH4+ and HCO3− to form carbamoyl phosphate. This reaction, catalyzed by carbamoyl phosphate synthetase I (CPS I), is the committed reaction of the urea cycle. Although carbamoyl phosphate is a simple molecule, its energy-
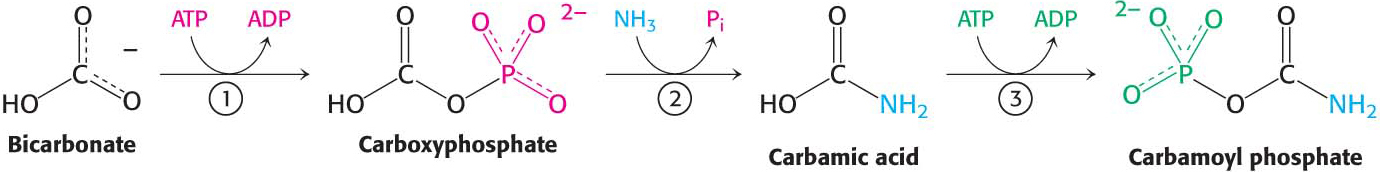
The reaction begins with the phosphorylation of HCO3− to form carboxyphosphate (1), which then reacts with ammonia to form carbamic acid (2). Finally, a second molecule of ATP phosphorylates carbamic acid to carbamoyl phosphate (3). The consumption of two molecules of ATP makes this synthesis of carbamoyl phosphate irreversible. Note that NH3, because it is a strong base, normally exists as NH4+ in aqueous solution. However, the carbamoyl phosphate synthetase deprotonates the ion and uses NH3 as a substrate.
Carbamoyl Phosphate Synthetase Is the Key Regulatory Enzyme for Urea Synthesis
Carbamoyl phosphate synthetase is regulated allosterically so that it is maximally active when amino acids are being metabolized for fuel use. The allosteric regulator N-
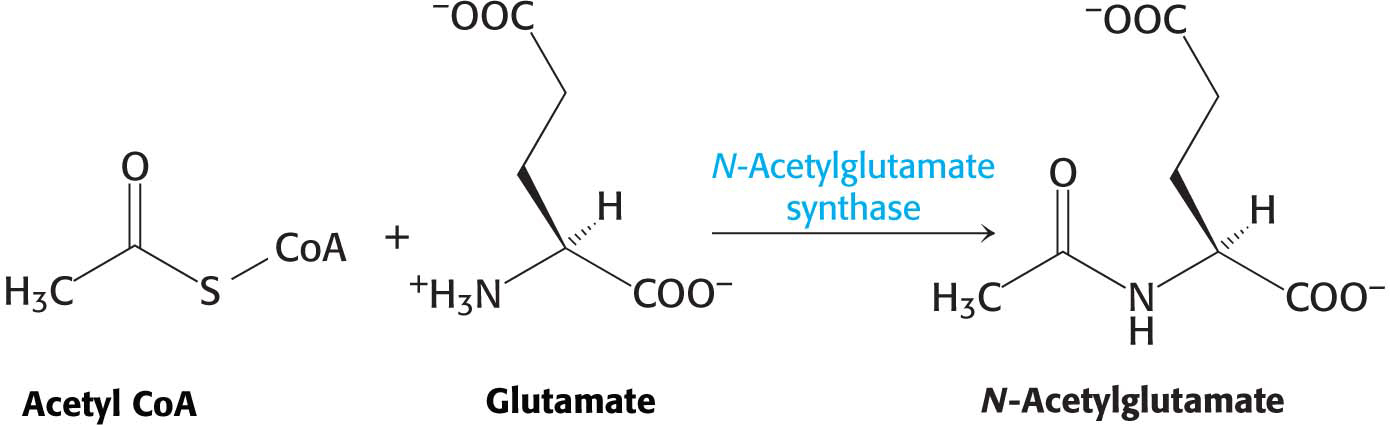
N-acetylglutamate synthase is itself activated by arginine. Thus, NAG is synthesized when amino acids, as represented by arginine and glutamate, are readily available, and carbamoyl phosphate synthetase is then activated to process the generated ammonia.
Carbamoyl Phosphate Reacts with Ornithine to Begin the Urea Cycle
The carbamoyl group of carbamoyl phosphate is transferred to ornithine to form citrulline, in a reaction catalyzed by ornithine transcarbamoylase:
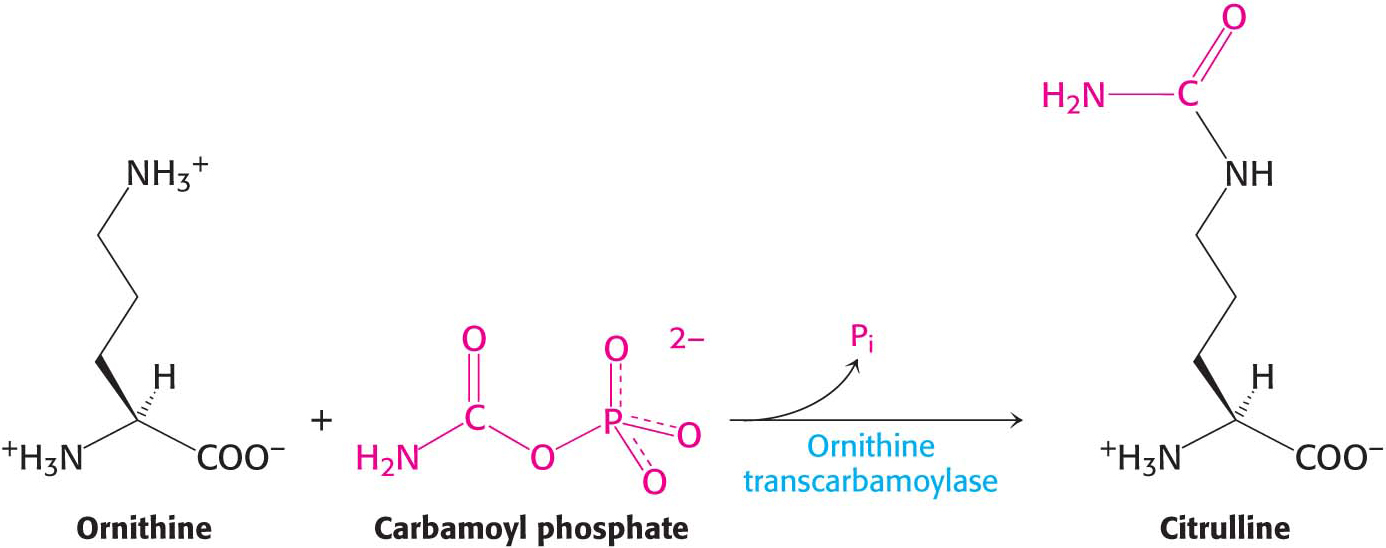
Ornithine and citrulline are amino acids, but they are not among the “alphabet” of 20 amino acids used as the building blocks of proteins. The formation of NH4+ by glutamate dehydrogenase, its incorporation into carbamoyl phosphate, and the subsequent synthesis of citrulline take place in the mitochondrial matrix. In contrast, the next three reactions of the urea cycle, which lead to the formation of urea, take place in the cytoplasm.
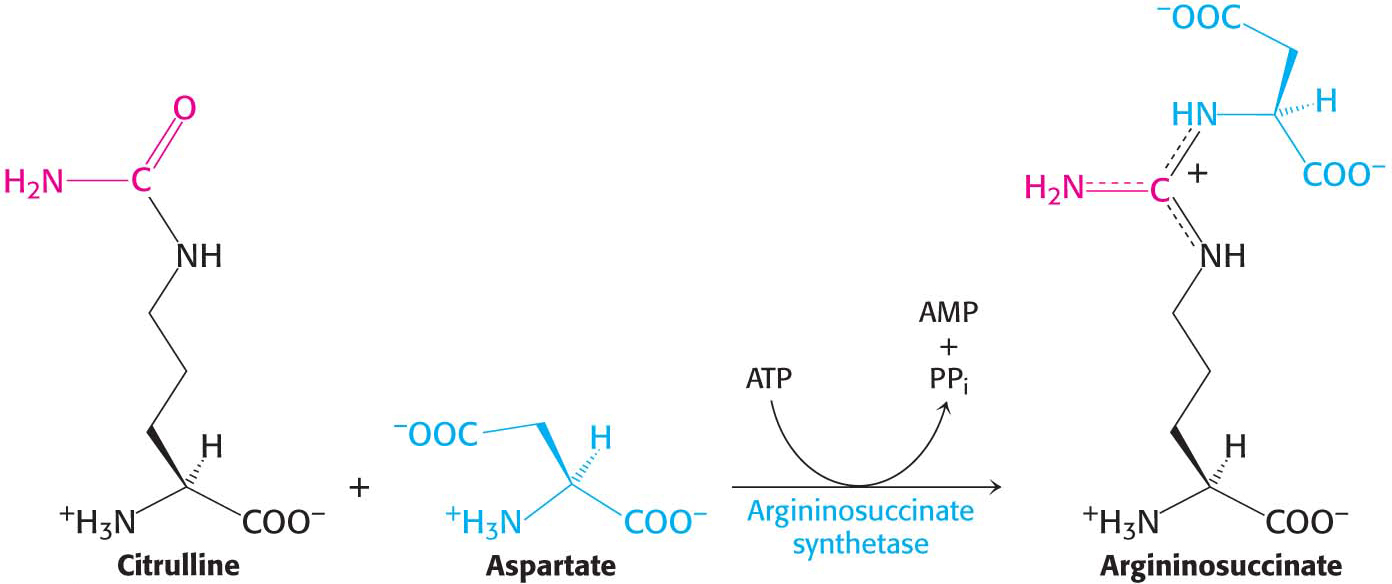
Citrulline, transported to the cytoplasm in exchange for ornithine, condenses with aspartate, the donor of the second amino group of urea. This synthesis of argininosuccinate, catalyzed by argininosuccinate synthetase, is driven by the cleavage of ATP into AMP and pyrophosphate and by the subsequent hydrolysis of pyrophosphate:
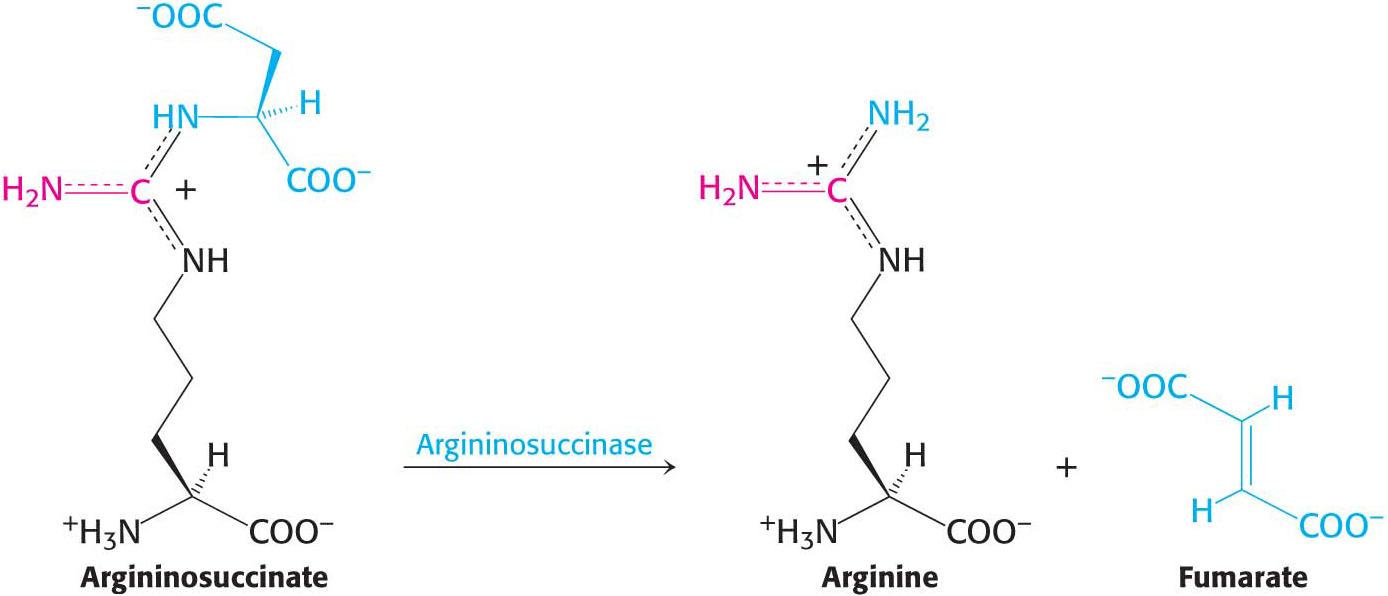
Argininosuccinase (also called argininosuccinate lyase) then cleaves argininosuccinate into arginine and fumarate. Thus, the carbon skeleton of aspartate is preserved in the form of fumarate:
DID YOU KNOW?
In ancient Rome, urine was a valuable commodity and was used to brighten togas. Vessels were placed on street corners to encourage passersby to urinate into them. Bacteria degraded the urea, releasing ammonium ion, which acted as a bleaching agent.
QUICK QUIZ 2
What are the immediate biochemical sources for the two nitrogen atoms in urea?
Carbamoyl phosphate and aspartate.
Finally, arginine is hydrolyzed to generate urea and ornithine in a reaction catalyzed by arginase. Ornithine is then transported back into a mitochondrion to begin another cycle. The urea is excreted. Indeed, human beings excrete about 10 kg (22 pounds) of urea per year.
The Urea Cycle Is Linked to Gluconeogenesis
The stoichiometry of urea synthesis is
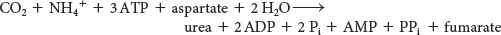
Pyrophosphate is rapidly hydrolyzed, and so the equivalent of four molecules of ATP are consumed in these reactions to synthesize one molecule of urea. The synthesis of fumarate by the urea cycle is important because it is a precursor for glucose synthesis (Figure 30.3). Fumarate is hydrated to malate, an intermediate of the citric acid cycle, which is in turn oxidized to oxaloacetate. Oxaloacetate can be converted into glucose by gluconeogenesis or transaminated to aspartate.
DID YOU KNOW?
The urea cycle generates enough arginine to meet the needs of a human adult, and so, for adults, arginine is not an essential amino acid. Children, however, need more building materials for their growing bodies and require arginine in the diet.

 CLINICAL INSIGHT
CLINICAL INSIGHTMetabolism in Context: Inherited Defects of the Urea Cycle Cause Hyperammonemia
The synthesis of urea in the liver is the major route of removal of NH4+. Urea cycle disorders occur with a prevalence of about 1 in 15,000 births. A blockage of carbamoyl phosphate synthesis or of any of the four steps of the urea cycle has devastating consequences because there is no alternative pathway for the synthesis of urea. All defects in the urea cycle lead to an elevated level of NH4+ in the blood (hyperammonemia). Some of these genetic defects become evident a day or two after birth, when the afflicted infant becomes lethargic and vomits periodically. Coma and irreversible brain damage may soon follow, a condition called hepatic encephalopathy.
Excessive alcohol consumption also can result in hyperammonemia. Earlier, we examined the effects of ethanol consumption on the liver. Much of the damage is due to the excessive production of NADH. Liver damage from excessive ethanol consumption takes place in three stages. In stage one, a fatty liver develops. In stage two—
Why are high levels of NH4+ toxic? The answer to this question is not yet known. Recent work, however, suggests that NH4+ may inappropriately activate a sodium–
 BIOLOGICAL INSIGHT
BIOLOGICAL INSIGHTHibernation Presents Nitrogen Disposal Problems
A variety of animals hibernate and, during hibernation, biochemical pathways continue to function to keep the animal alive. Let’s consider a hibernating bear, which may sleep for 3 to 5 months. During this time, the bear becomes a closed, self-
The nitrogen is still salvaged to produce urea, which is passed to the bladder. In the hibernating bear, the urea is then absorbed into the blood and released into the intestine. Bacteria in the intestine hydrolyze the urea, generating NH4+, which is used to synthesize amino acids and proteins. When the microorganisms die, the released amino acids are absorbed by the bear and used for biosynthesis. Obviously, both the bear and the bacteria benefit. The bear’s urea is disposed of and returned as needed amino acids, and the bacteria have a nitrogen source for their biosynthesis. Interestingly, some of the bacteria in the bear can use the hydrolysis of urea to generate an electrochemical gradient that is used to synthesize ATP. The bear may be hibernating, but the bear and its inhabitants are a cauldron of biochemical activity.
 BIOLOGICAL INSIGHT
BIOLOGICAL INSIGHTUrea Is Not the Only Means of Disposing of Excess Nitrogen
As stated earlier, most terrestrial vertebrates are ureotelic; they excrete excess nitrogen as urea. However, urea is not the only excretable form of nitrogen. Ammoniotelic organisms, such as aquatic vertebrates and invertebrates, release nitrogen as NH4+ and rely on the aqueous environment to dilute this toxic substance. So, such organisms do not need to process nitrogen, as in the urea cycle, but can excrete it as it is removed from amino acids. Interestingly, normally ammoniotelic lungfish become ureotelic in times of drought, when they live out of the water.
Both ureotelic and ammoniotelic organisms depend on water, to varying degrees, for nitrogen excretion. In contrast, uricotelic organisms, which secrete nitrogen as the purine uric acid, require little water. The disposal of excess nitrogen as uric acid is especially valuable in animals, such as birds, that produce eggs having impermeable membranes that accumulate waste products. The pathway for nitrogen excretion clearly depends on the habitat of the organism.