
31.2 Amino Acids Are Made from Intermediates of Major Pathways
✓ 4 Identify the sources of carbon atoms for amino acid synthesis.
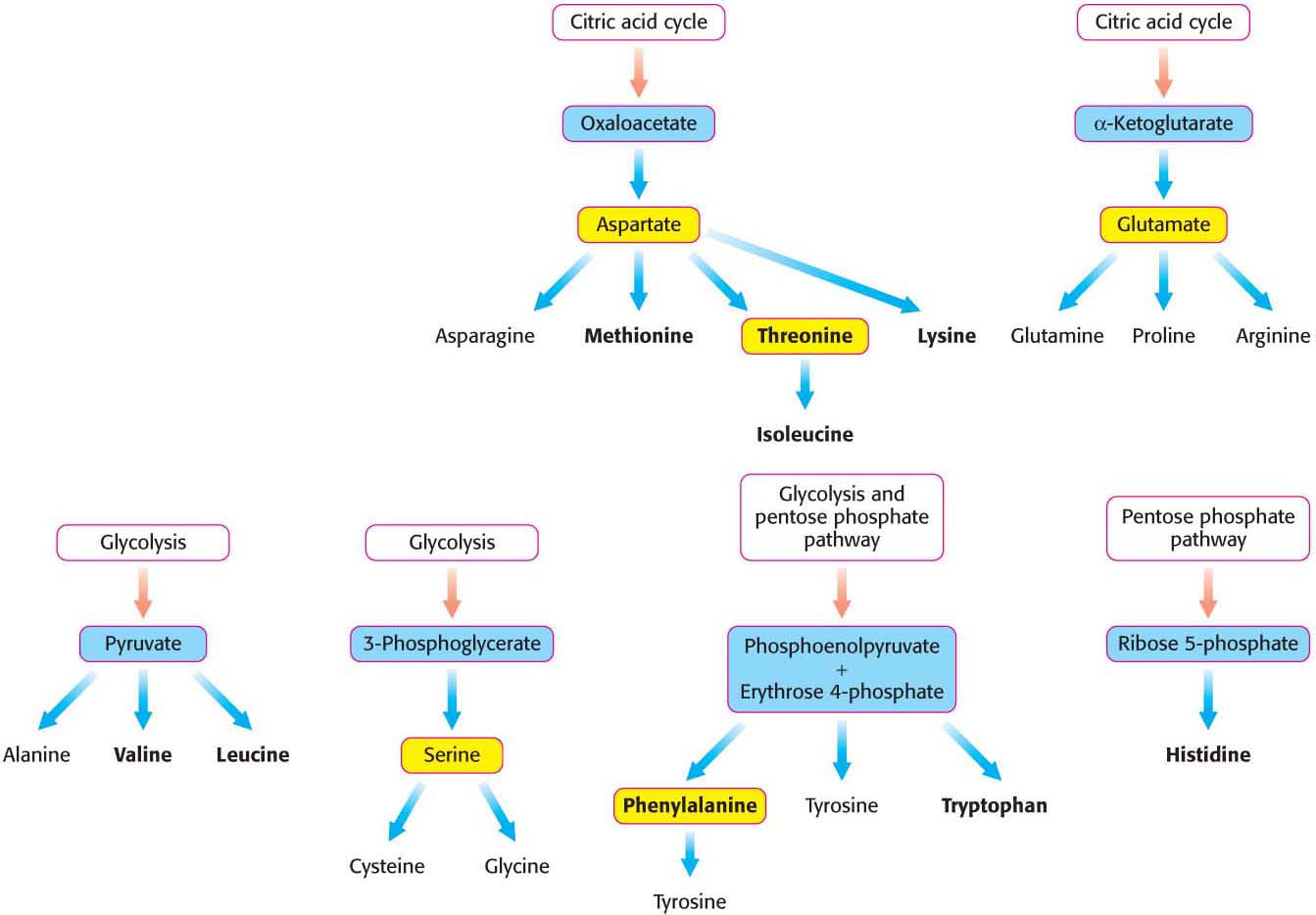
Thus far, we have considered the conversion of N2 into NH4+ and the assimilation of NH4+ into glutamate and glutamine. We now turn to the biosynthesis of the other amino acids. The pathways for the biosynthesis of amino acids are diverse. However, they have an important common feature: their carbon skeletons come from only a few sources: intermediates of glycolysis, the citric acid cycle, or the pentose phosphate pathway. On the basis of these starting materials, amino acids can be grouped into six biosynthetic families (Figure 31.5).
Human Beings Can Synthesize Some Amino Acids but Must Obtain Others from the Diet
NUTRITION FACTS

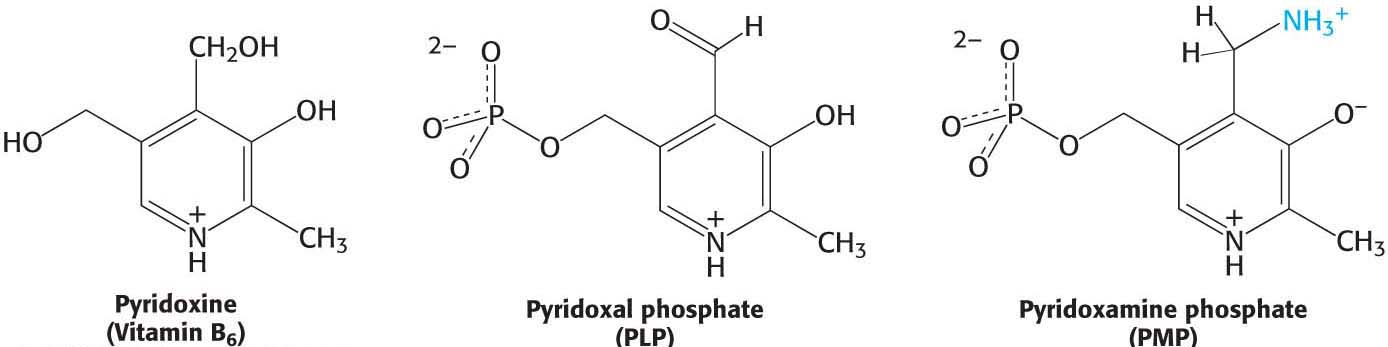
Pyridoxine (vitamin B6) A precursor to pyridoxal phosphate (PLP) and pyridoxamine phosphate (PMP), pyridoxine also serves as a cofactor in a host of enzymes including transaminases. Pyridoxine is found in plants, whereas PLP and PMP are plentiful in salmon and the white meat of poultry. A deficiency of vitamin B6, which is rare in the United States, results in fatigue, fissures at the corners of the mouth (cheilosis), inflammation of the tongue (glossitis), and inflammation of the lining of the mouth (stomatitis).
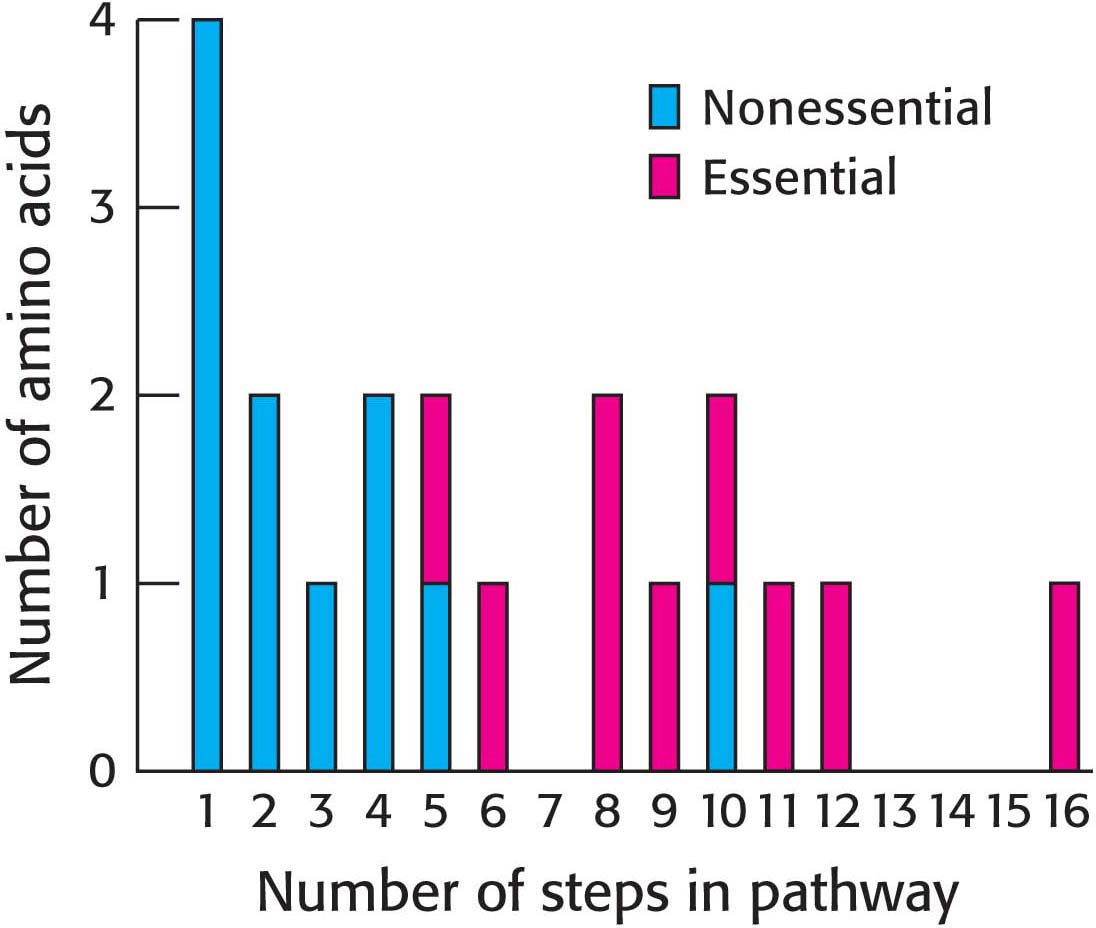
Most microorganisms such as E. coli can synthesize the entire basic set of 20 amino acids, but human beings can make only 11 of them. The amino acids that must be supplied in the diet are called essential amino acids, whereas the others, which can be synthesized if dietary content is insufficient, are termed nonessential amino acids (Table 3.2). A deficiency of even one amino acid compromises the ability of an organism to synthesize all of the proteins required for life.
The nonessential amino acids are synthesized by quite simple reactions, whereas the pathways for the formation of the essential amino acids are quite complex. For example, the nonessential amino acids alanine and aspartate are synthesized in a single step from pyruvate and oxaloacetate, respectively. In contrast, the pathways for the essential amino acids require from 5 to 16 steps (Figure 31.6). The sole exception to this pattern is arginine, inasmuch as the synthesis of this nonessential amino acid de novo requires 10 steps. Typically, though, arginine is made in only 3 steps from ornithine as part of the urea cycle. Tyrosine, classified as a nonessential amino acid because it can be synthesized in 1 step from phenylalanine, requires 10 steps to be synthesized from scratch and is essential if phenylalanine is not abundant. We will examine some important features of amino acid synthesis.
Some Amino Acids Can Be Made by Simple Transamination Reactions
Three α-ketoacids—
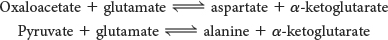
Transaminations are carried out by pyridoxal phosphate-
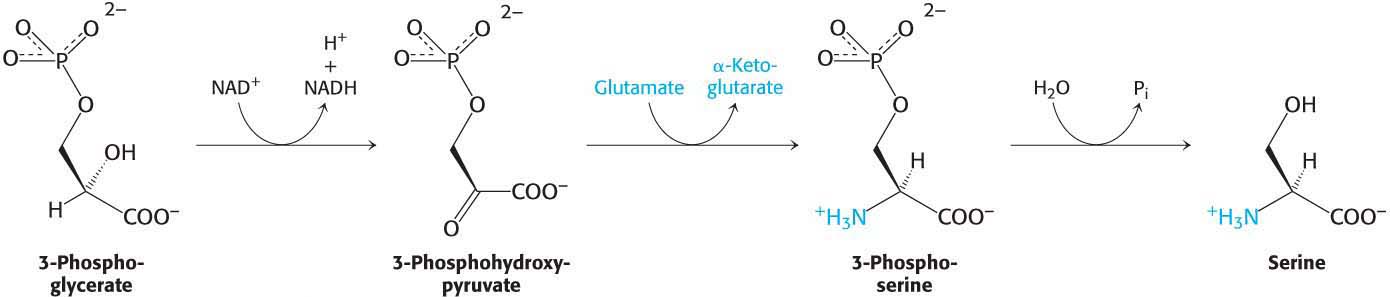
Serine, Cysteine, and Glycine Are Formed from 3-Phosphoglycerate
Serine is synthesized from 3-
NUTRITION FACTS

Folic acid (vitamin B9) Another of the B vitamins, folic acid is especially important for growth, and a lack of folic acid during prenatal development results in spinal-
 CLINICAL INSIGHT
CLINICAL INSIGHTTetrahydrofolate Carries Activated One-Carbon Units
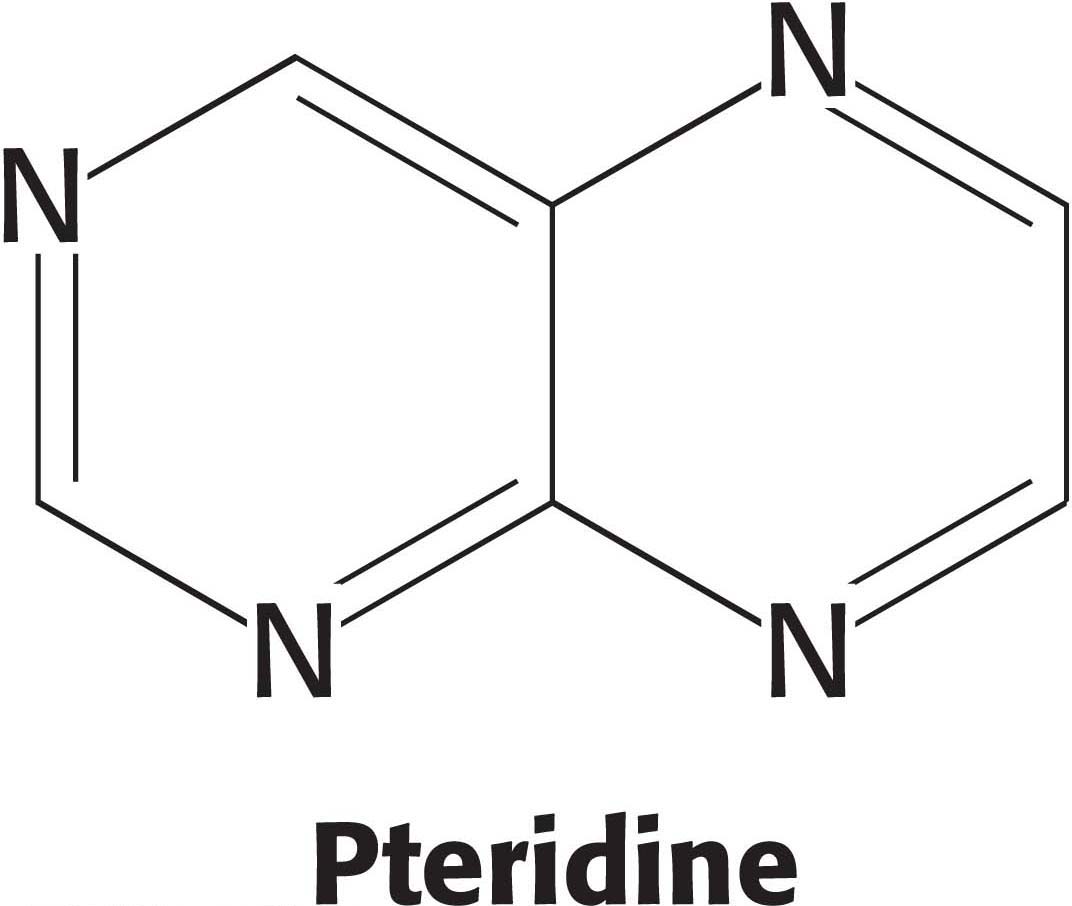
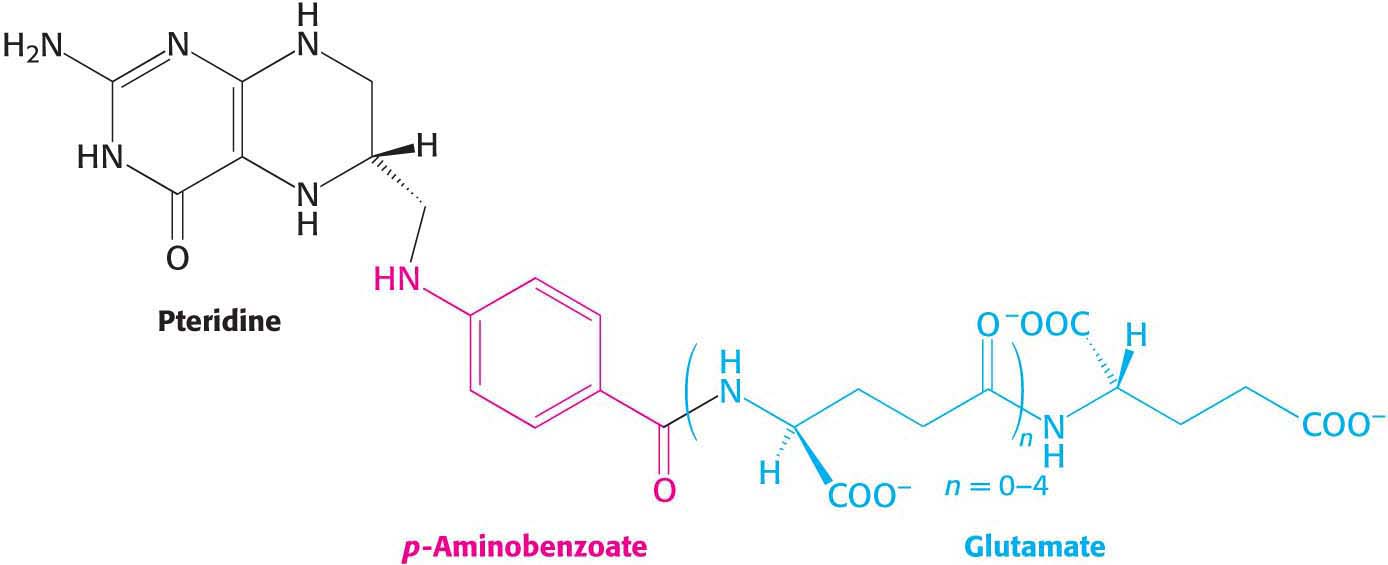
Tetrahydrofolate is a coenzyme essential for the synthesis of many amino acids and nucleotides. This coenzyme, a highly versatile carrier of activated one-
The one-
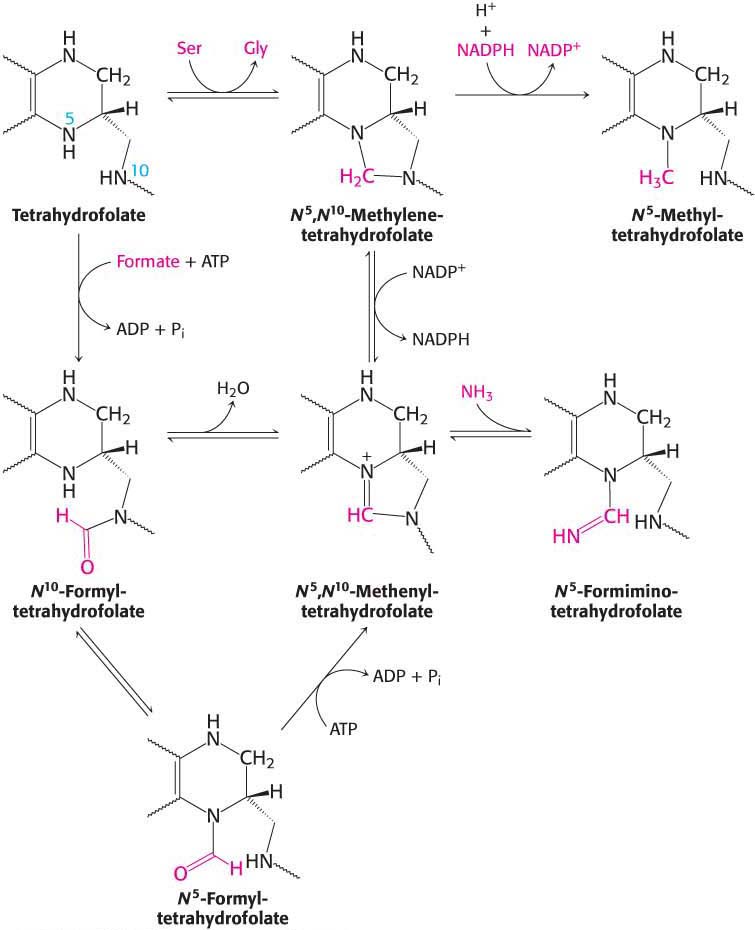
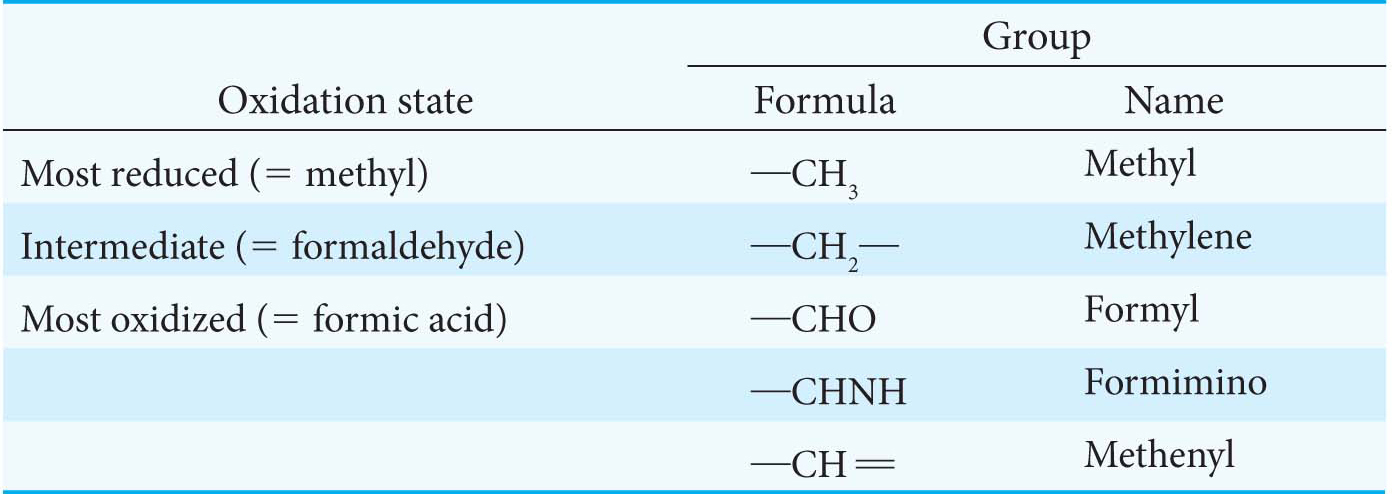
More oxidized forms carry a formyl, formimino, or methenyl group. The fully oxidized one-
Tetrahydrofolate, derived from folic acid (vitamin B9), plays an especially important role in the development of the fetal nervous system during early pregnancy. Folic acid deficiency can result in failure of the neural tube to close, which results in conditions such as spina bifida (defective closure of the vertebral column) and anencephaly (lack of a brain). The neural tube closes by about the 28th day of pregnancy, usually before a woman knows that she is pregnant. Consequently, some physicians recommend that all women of childbearing age take folic acid supplements.
S-Adenosylmethionine Is the Major Donor of Methyl Groups
Tetrahydrofolate can carry a methyl group on its N-
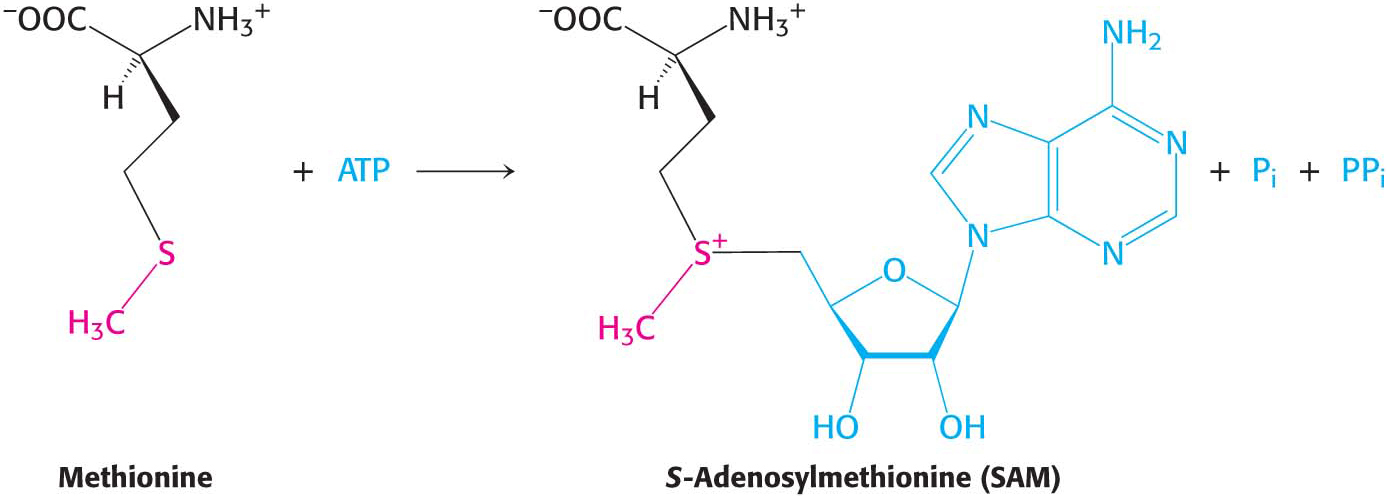
The methyl group of the methionine unit is activated by the positive charge on the adjacent sulfur atom, which makes the molecule much more reactive than N5-methyltetrahydrofolate. Recall that S-adenosylmethionine is an activated methyl donor in the synthesis of phosphatidylcholine from phosphatidylethanolamine.
The synthesis of S-adenosylmethionine is unusual in that the triphosphate group of ATP is split into pyrophosphate and orthophosphate; the pyrophosphate is subsequently hydrolyzed to two molecules of Pi. S-Adenosylhomocysteine is formed when the methyl group of S-adenosylmethionine is transferred to an acceptor. S-Adenosylhomocysteine is then hydrolyzed to homocysteine and adenosine:
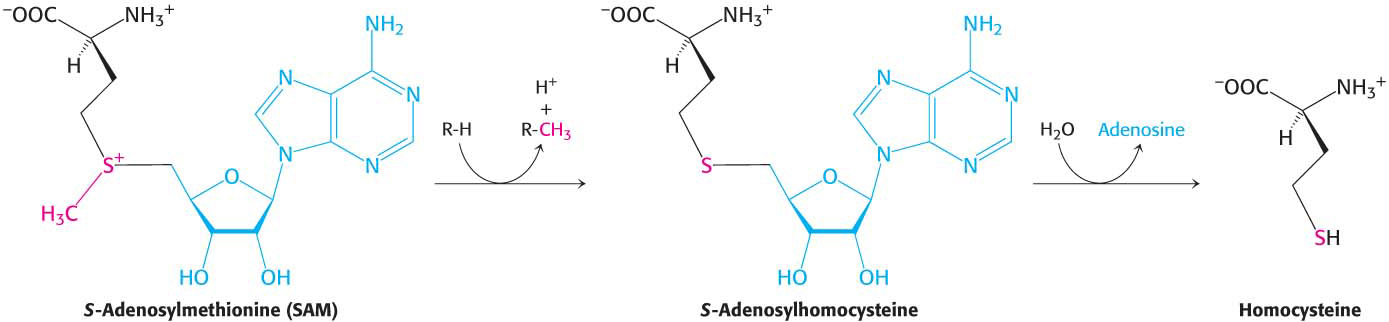
Methionine can be regenerated by the transfer of a methyl group to homocysteine from N5-methyltetrahydrofolate, a reaction catalyzed by methionine synthase (also known as homocysteine methyltransferase). The coenzyme that mediates this transfer of a methyl group is methylcobalamin, derived from vitamin B12.
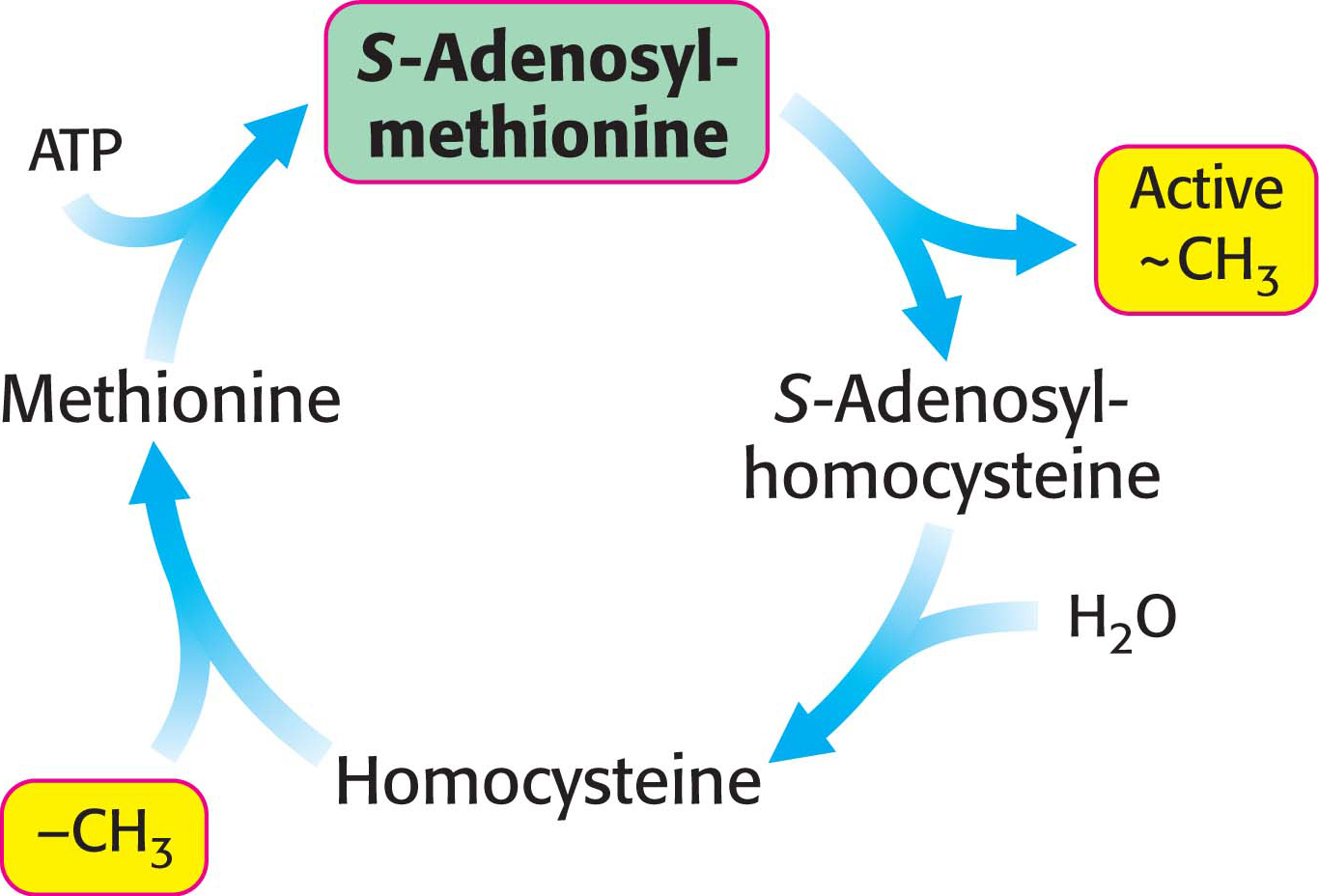
These reactions constitute the activated methyl cycle (Figure 31.9). Methyl groups enter the cycle in the conversion of homocysteine into methionine and are then made highly reactive by the addition of an adenosyl group. The high transfer potential of the methyl group of S-adenosylmethionine enables it to be transferred to a wide variety of acceptors.
QUICK QUIZ 2
Identify the six biosynthetic families of amino acids.
Asparagine, aspartate, methionine, threonine, isoleucine, and lysine constitute one family derived from oxaloacetate. Phenylalanine, tyrosine, and tryptophan constitute another family derived from phosphoenolpy ruvate and erythrose 4-
 CLINICAL INSIGHT
CLINICAL INSIGHTHigh Homocysteine Levels Correlate with Vascular Disease
People with elevated serum levels of homocysteine (homocysteinemia) or the disulfide-
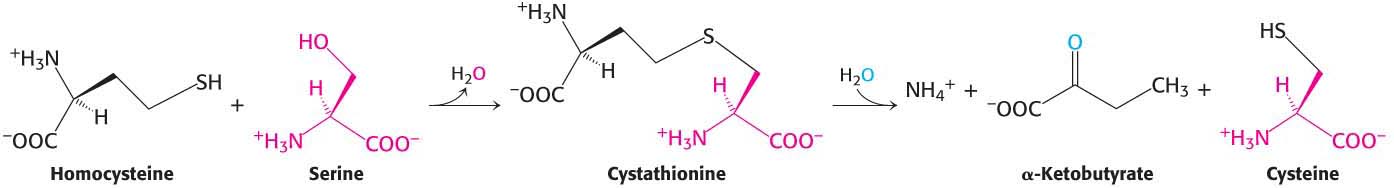
High levels of homocysteine appear to damage cells lining blood vessels and to increase the growth of vascular smooth muscle. The amino acid raises oxidative stress as well and has also been implicated in the development of type 2 diabetes. The molecular basis of homocysteine’s action has not been clearly identified, but may result from stimulation of the inflammatory response. Vitamin treatments are sometimes effective in reducing homocysteine levels in some people. These treatments maximize the activity of the two major metabolic pathways processing homocysteine. Pyridoxal phosphate, a vitamin B6 derivative, is necessary for the activity of cystathionine β-synthase, and tetrahydrofolate, as well as vitamin B12, are required for the methylation of homocysteine to methionine.