
31.3 Feedback Inhibition Regulates Amino Acid Biosynthesis
The rate of synthesis of amino acids depends mainly on the amounts of the biosynthetic enzymes and on their activities. We now consider the control of enzymatic activity. The regulation of enzyme synthesis will be discussed in Section 15.
The Committed Step Is the Common Site of Regulation
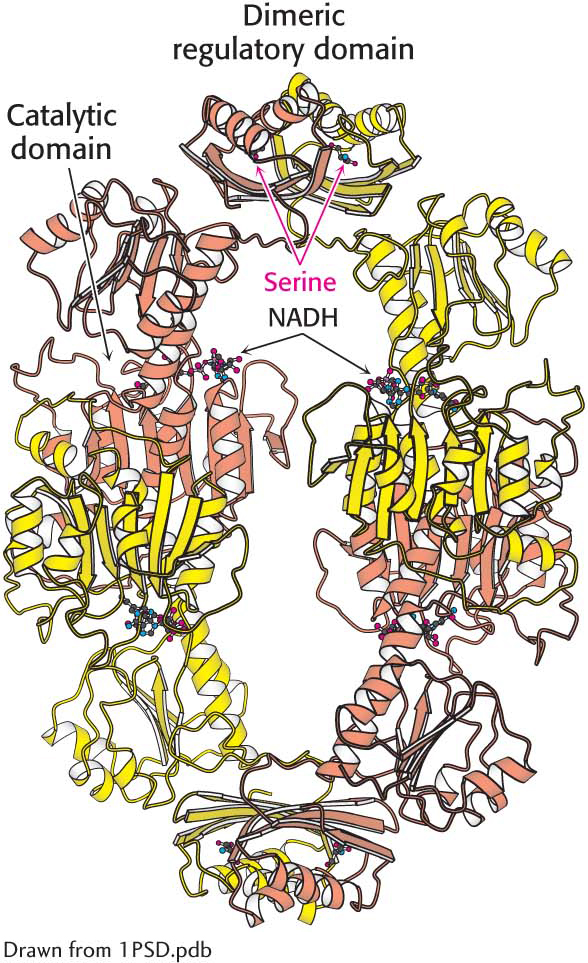
 The structure of 3-
The structure of 3-As we have seen in every metabolic pathway that we have studied so far, the first irreversible reaction, or the committed step, is usually an important regulatory site. The final product of the pathway (Z) often inhibits the enzyme that catalyzes the committed step (A → B).
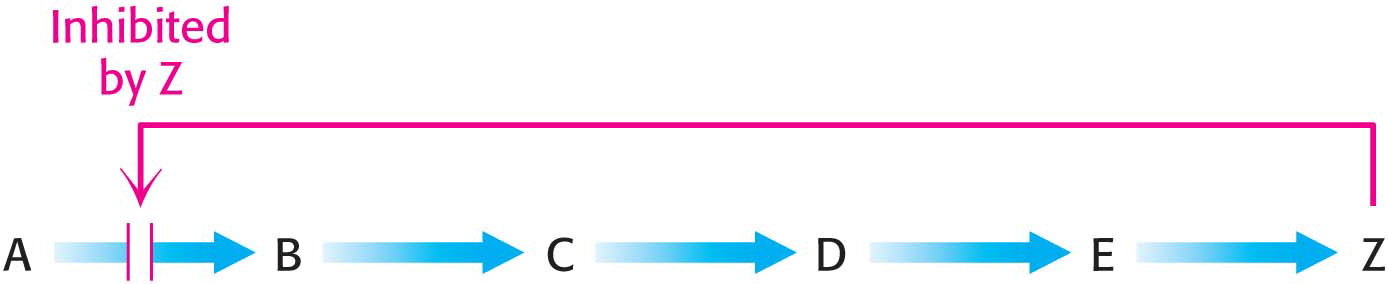
This kind of control is essential for the conservation of building blocks and metabolic energy. Consider the biosynthesis of serine. The committed step in this pathway is the oxidation of 3-
Branched Pathways Require Sophisticated Regulation
The regulation of branched pathways is more complicated because the concentration of two products must be accounted for. In fact, several intricate feedback mechanisms have been found in branched biosynthetic pathways.
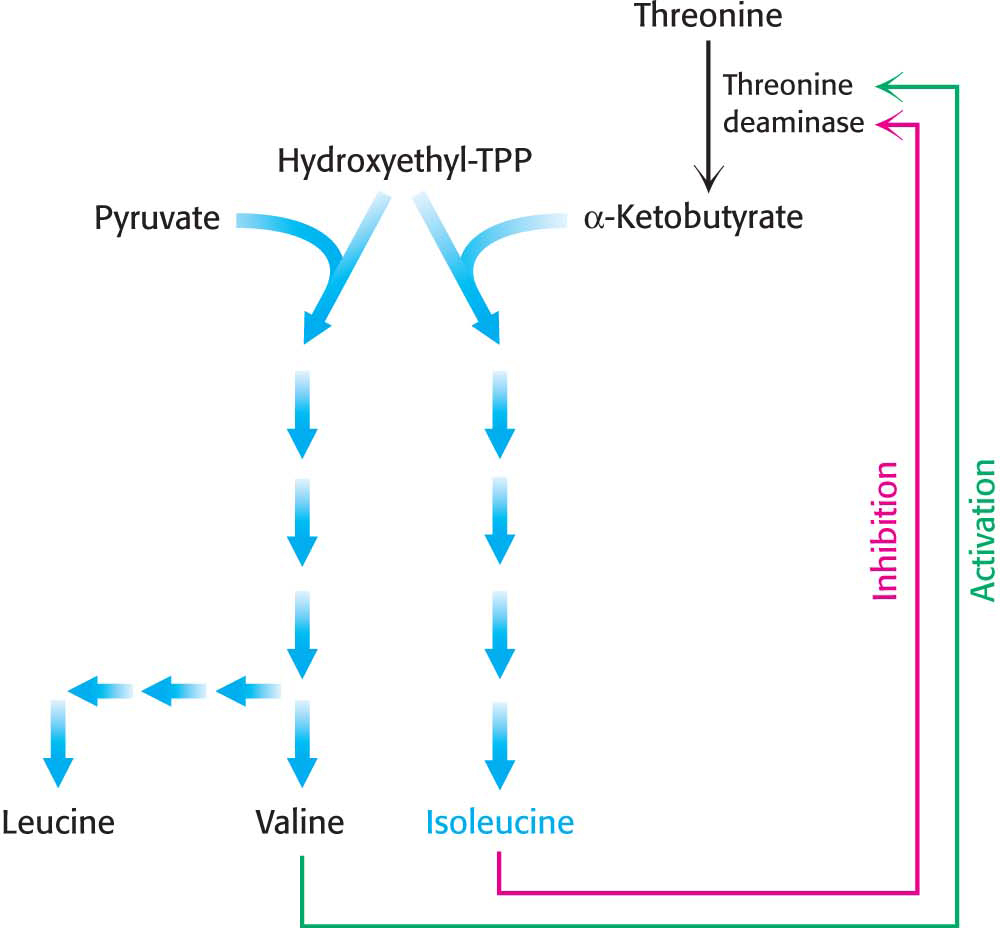
Feedback inhibition and activationTwo pathways with a common initial step may each be inhibited by its own product and activated by the product of the other pathway. Consider, for example, the biosynthesis of the amino acids valine, leucine, and isoleucine (Figure 31.11). A common intermediate, hydroxyethyl thiamine pyrophosphate (hydroxyethyl-
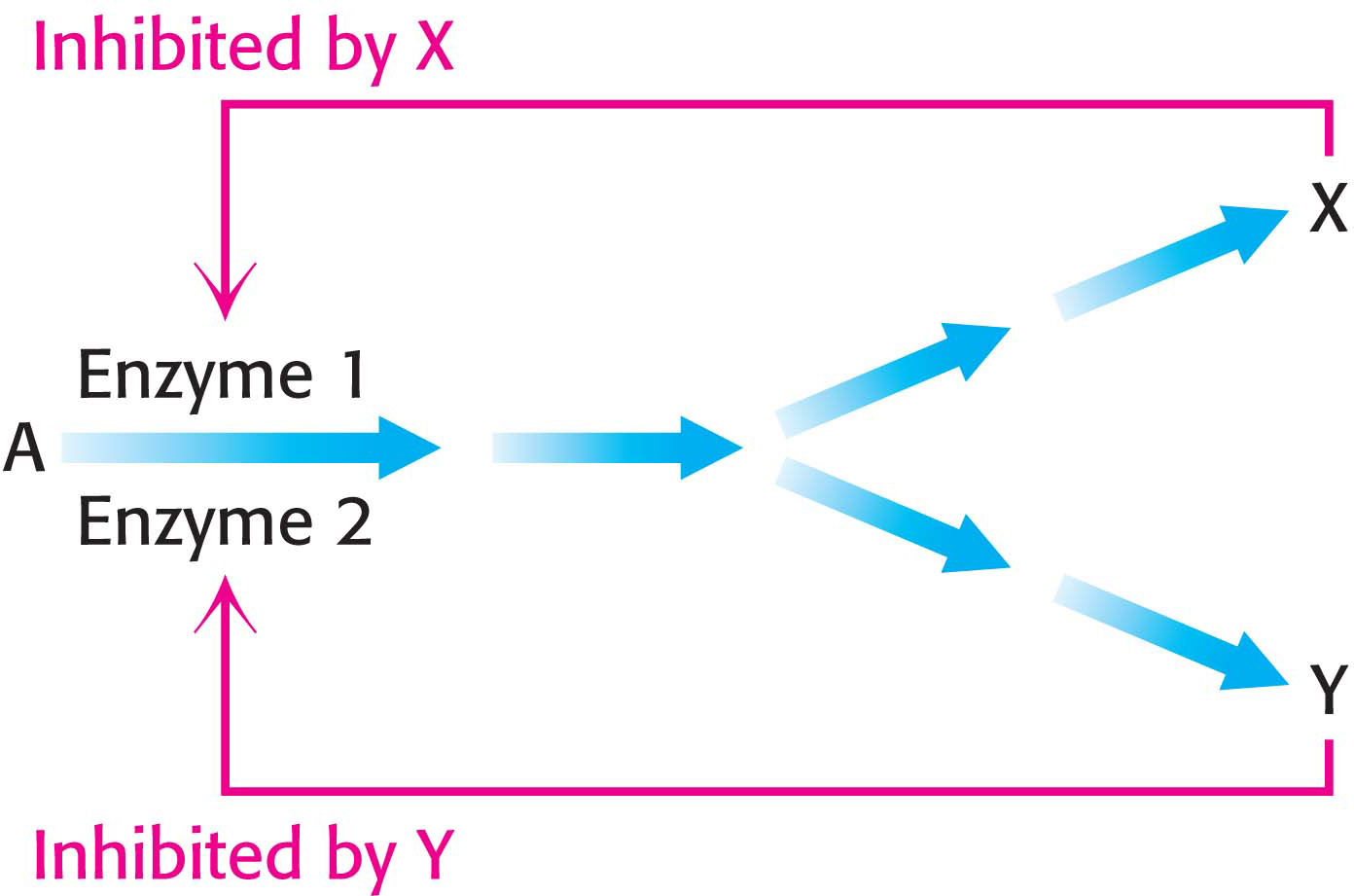
Enzyme multiplicityThe committed step can be catalyzed by two or more enzymes with different regulatory properties, a strategy referred to as enzyme multiplicity (Figure 31.12). For example, the phosphorylation of aspartate is the committed step in the biosynthesis of threonine, methionine, and lysine. Three distinct aspartokinases catalyze this reaction in E. coli. Although the mechanisms of catalysis are essentially identical, their activities are regulated differently: one enzyme is not subject to feedback inhibition, another is inhibited by threonine, and the third is inhibited by lysine.
Cumulative feedback inhibitionA common step is partly inhibited by each of the final products, acting independently, in cumulative feedback inhibition. The regulation of glutamine synthetase in E. coli is a striking example of this inhibition. Recall that glutamine is synthesized from glutamate, NH4+, and ATP. Glutamine synthetase regulates the flow of nitrogen and hence plays a key role in controlling bacterial metabolism. The amide group of glutamine is a source of nitrogen in the biosyntheses of a variety of compounds, such as tryptophan, histidine, carbamoyl phosphate, glucosamine 6-