
32.2 The Pyrimidine Ring Is Assembled and Then Attached to a Ribose Sugar
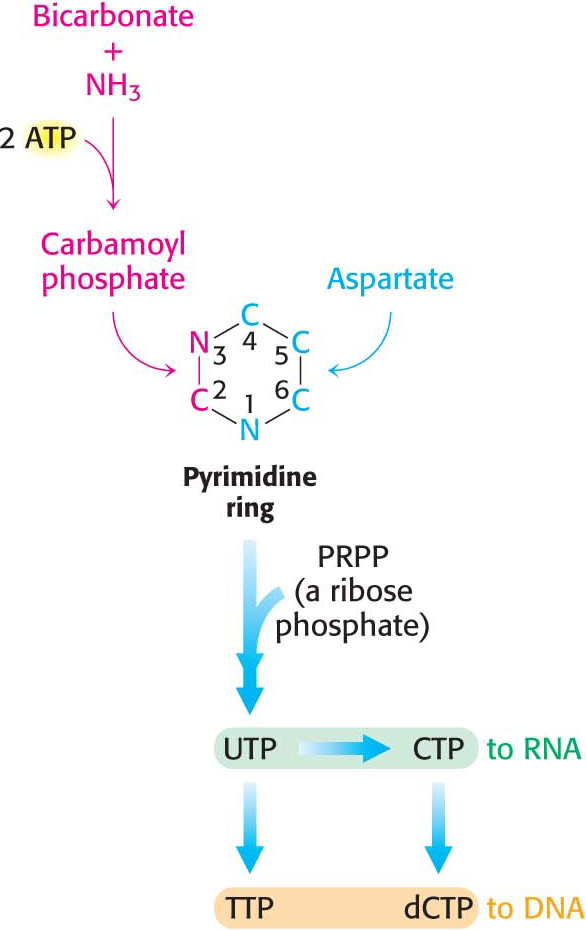
The precursors of pyrimidine rings are bicarbonate, aspartic acid, and ammonia usually produced from the hydrolysis of the side chain of glutamine. In the de novo synthesis of pyrimidines, the ring is synthesized first and then it is attached to ribose to form a pyrimidine nucleotide (Figure 32.3).
The first step in de novo pyrimidine biosynthesis is the synthesis of carbamoyl phosphate from bicarbonate and ammonia in a three-
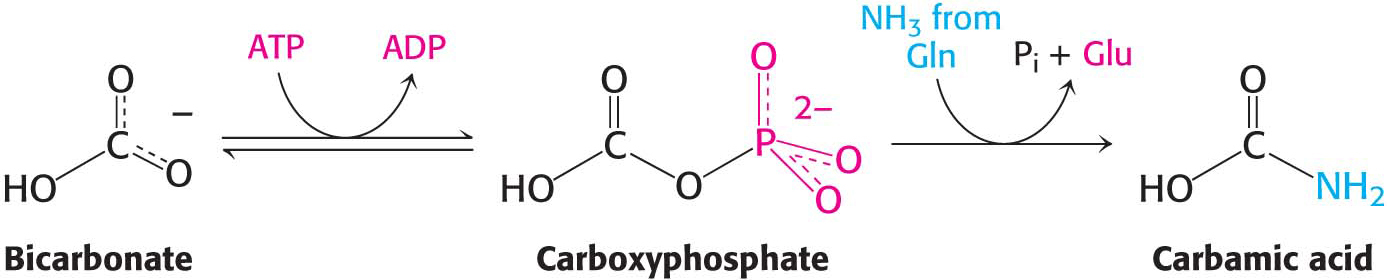
In the first step of the carbamoyl phosphate synthesis pathway, bicarbonate is phosphorylated by ATP to form carboxyphosphate, an activated form of CO2, and ADP. In the second step, glutamine is hydrolyzed by CPS to yield glutamate and ammonia, which then reacts with carboxyphosphate to form carbamic acid and inorganic phosphate:
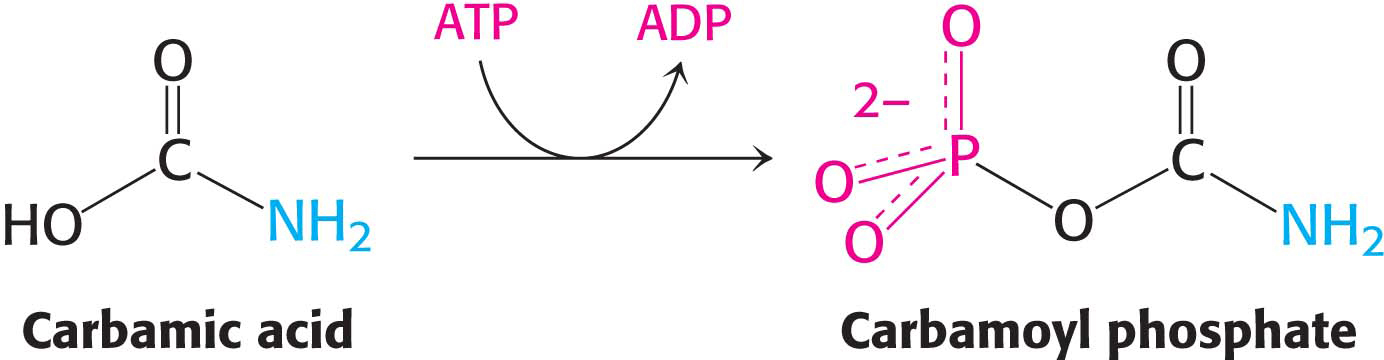
Carbamic acid is then phosphorylated by another molecule of ATP to form carbamoyl phosphate:
The reaction catalyzed by carbamoyl phosphate synthetase II (CPS II) is identical with that of carbamoyl phosphate synthetase I (CPS I) required for urea synthesis. CPS II, however, uses glutamine as a nitrogen source rather than free ammonia and does not require N-acetylglutamate.
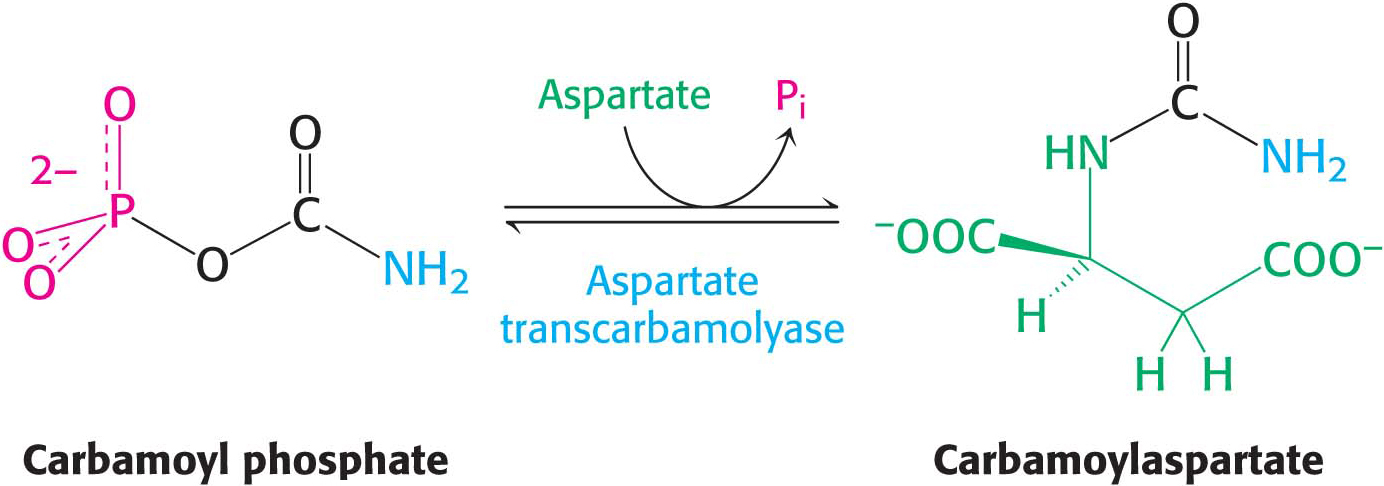
Carbamoyl phosphate reacts with the amino acid aspartate to form carbamoylaspartate in a reaction catalyzed by aspartate transcarbamoylase (ATCase). This allosteric enzyme regulates the synthesis of pyrimidine nucleotides, as we will see later.
Carbamoylaspartate then cyclizes to form dihydroorotate, a reaction catalyzed by dihydroorotase. Dihydroorotate is subsequently oxidized by NAD+ to form orotate:
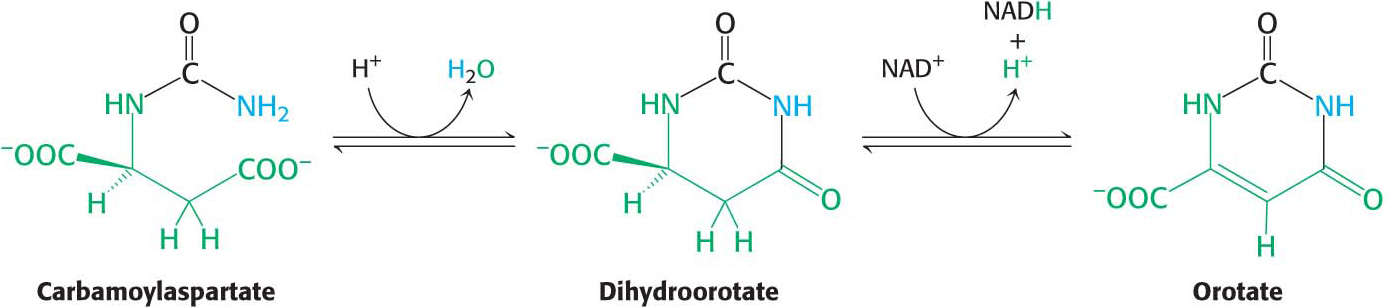
In mammals, three of the enzymes of orotate synthesis are part of a single large polypeptide chain called CAD, for carbamoyl phosphate synthetase, aspartate transcarbamoylase, and dihydroorotase.
At this stage, orotate couples to 5-
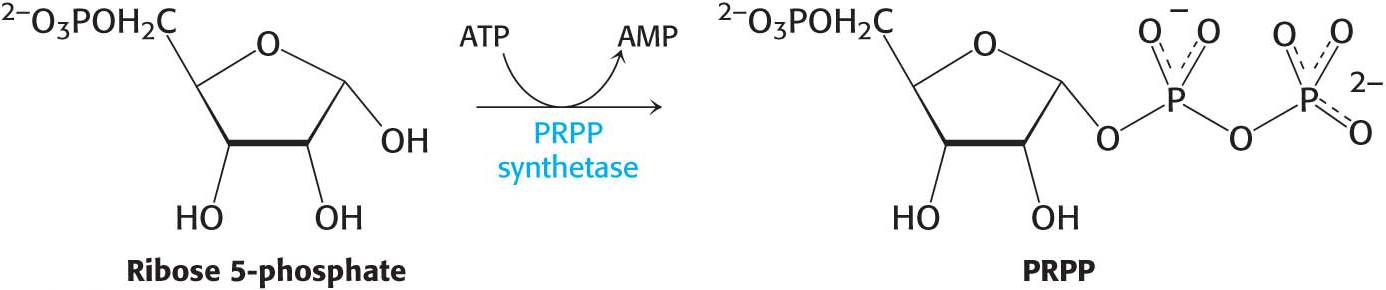
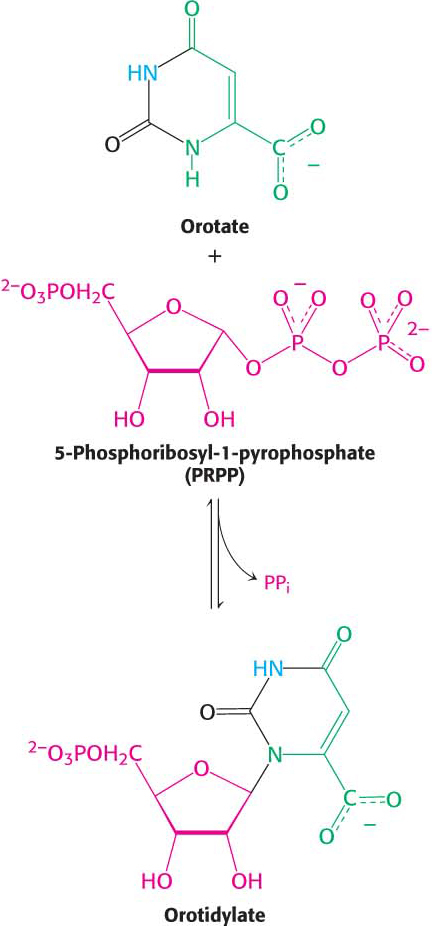
Orotate reacts with PRPP to form orotidylate, a pyrimidine nucleotide, in a reaction catalyzed by oroate phosphoribosyltransferase. The hydrolysis of pyrophosphate, as shown in the margin, renders the reaction irreversible. Orotidylate is then decarboxylated to form uridine monophosphate (UMP, or uridylate), one of two major pyrimidine nucleotides that are precursors of RNA. This reaction is catalyzed by orotidylate decarboxylase also called orotidine-
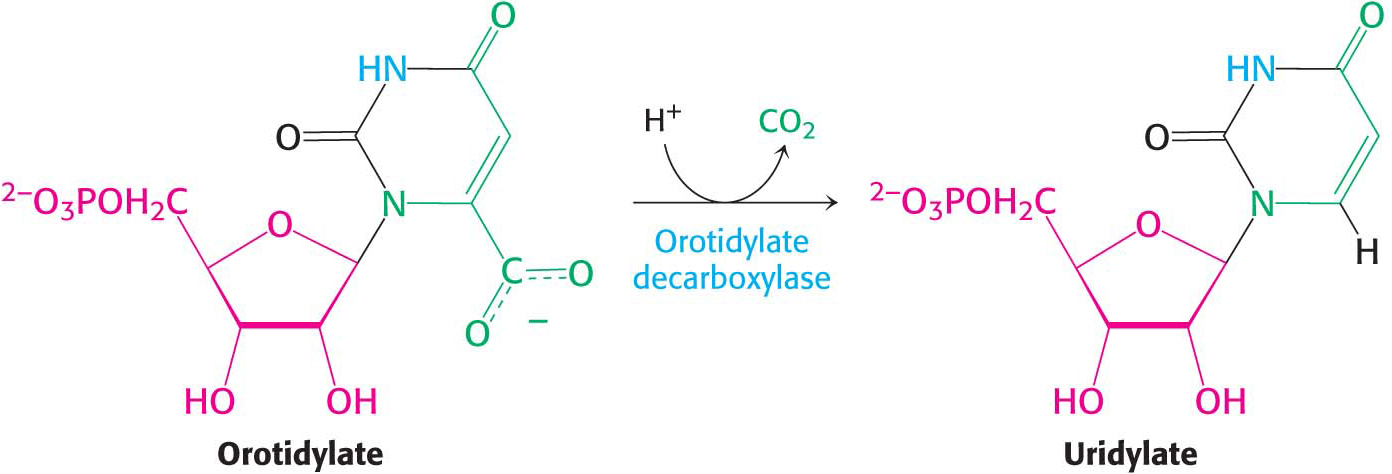
The phosphoribosyltransferase and decarboxylase activities are located on the same polypeptide chain, providing another example of a bifunctional enzyme. The bifunctional enzyme is called uridine monophosphate synthetase.
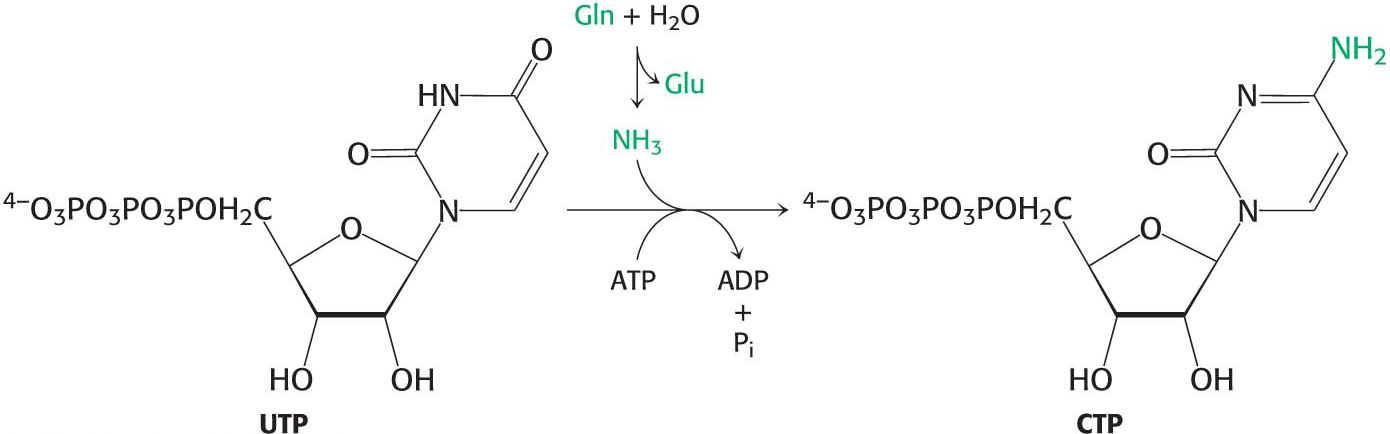
CTP Is Formed by the Amination of UTP
How is the other major pyrimidine ribonucleotide, cytidine monophosphate (CMP, or cytidylate), formed? It is synthesized from the uracil base of UMP, but UMP must be converted into UTP before the synthesis can take place.
Kinases Convert Nucleoside Monophosphates into Nucleoside Triphosphates
Recall that the diphosphates and triphosphates are the active forms of nucleotides in biosynthesis and energy conversions. Nucleoside monophosphates are converted into nucleoside triphosphates in a step-

Second, nucleoside diphosphates and triphosphates are interconverted by nucleoside diphosphate kinase, an enzyme that has broad specificity, in contrast with the monophosphate kinases. The letters X and Y can represent any of several ribonucleosides or even deoxyribonucleosides.

After uridine triphosphate has been formed, it can be transformed into cytidine triphosphate (CTP) by the replacement of a carbonyl group by an amino group. Like the synthesis of carbamoyl phosphate, this reaction requires ATP and uses glutamine as the source of the amino group. CTP can then be used in many biochemical processes, including RNA synthesis and the activation of molecules for phospholipid synthesis.
QUICK QUIZ 1
What are the precursors for the de novo synthesis of pyrimidines?
Bicarbonate, ammonia, and aspartate (Figure 32.3).
 CLINICAL INSIGHT
CLINICAL INSIGHTSalvage Pathways Recycle Pyrimidine Bases
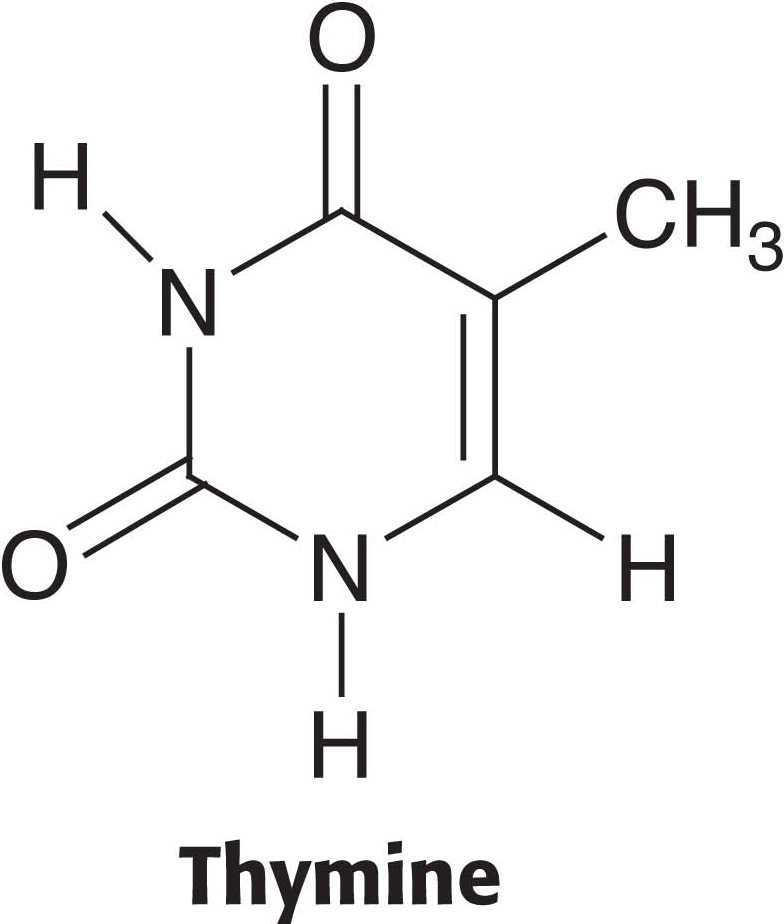
Pyrimidine bases can be recovered from the breakdown products of DNA and RNA by the use of salvage pathways. In these pathways, a preformed base is reincorporated into a nucleotide. We will consider the salvage pathway for the pyrimidine base thymine. Thymine is found in DNA and base-
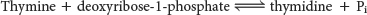
Thymidine is then converted into a nucleotide by thymidine kinase:
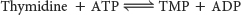
The activity of thymidine kinase fluctuates with the cell cycle, displaying peak activity during the S phase, when DNA synthesis takes place.
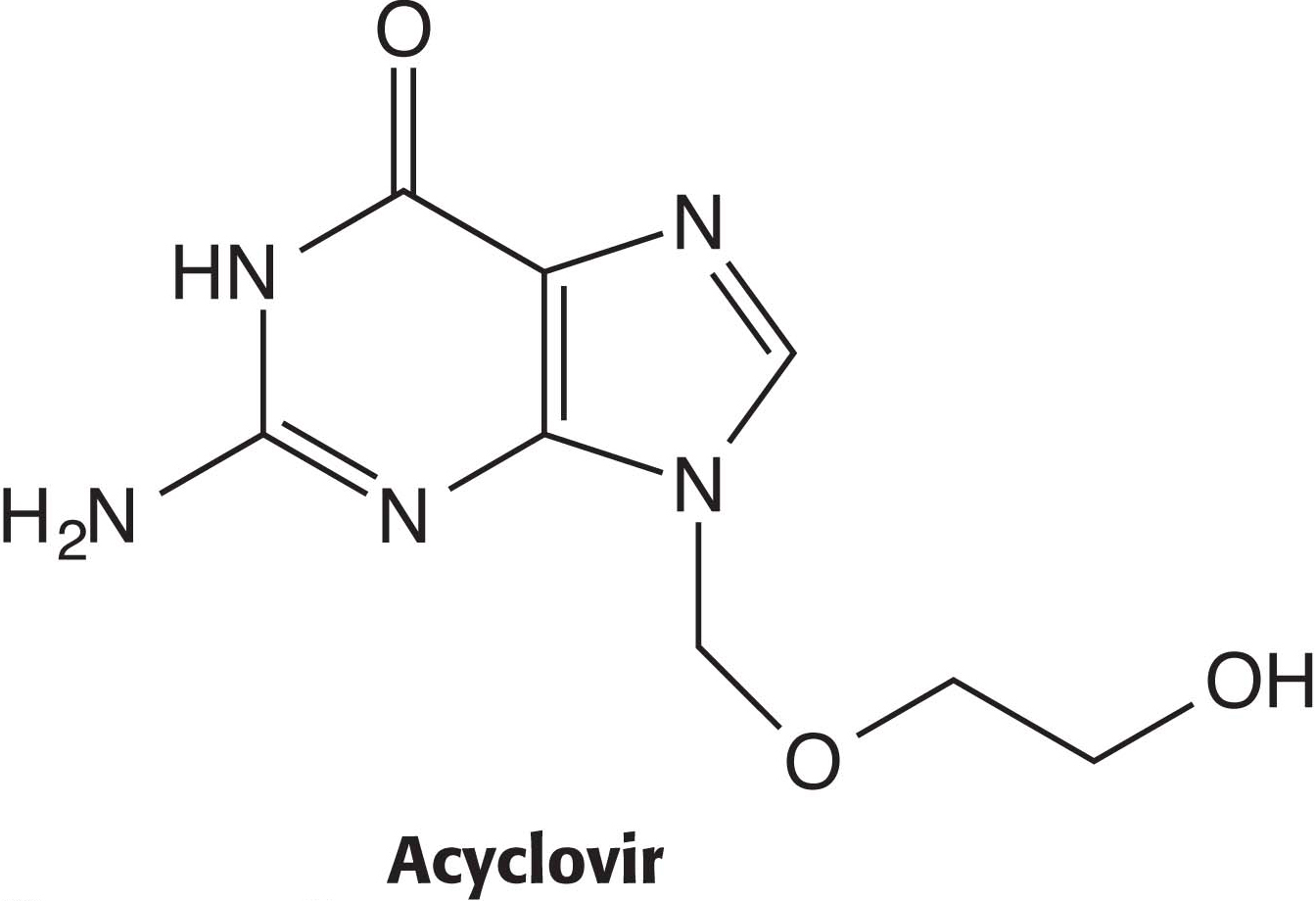
Viral thymidine kinase differs from the mammalian enzyme and thus provides a therapeutic target. For instance, herpes simplex infections are treated with acyclovir, which viral thymidine kinase converts into a suicide inhibitor that terminates DNA synthesis. As we will see shortly, thymidine kinase also plays a role in the de novo synthesis of thymidylate.