
32.4 Ribonucleotides Are Reduced to Deoxyribonucleotides

We now turn to the synthesis of deoxyribonucleotides. These precursors of DNA are formed by the reduction of ribonucleotides. The 2′-hydroxyl group on the ribose moiety is replaced by a hydrogen atom. The substrates are ribonucleoside diphosphates, and the ultimate reductant is NADPH (Figure 32.8). The same enzyme, ribonucleotide reductase, acts on all four ribonucleotides. The overall stoichiometry is
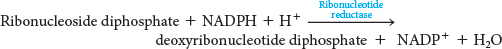
The reaction mechanism is more complex than implied by this equation. Ribonucleotide reductase catalyzes only the last step in this reaction. The reductase itself must be reduced to perform another catalytic cycle, and the electrons for this reduction come from NADPH, but not directly. One carrier of reducing power linking NADPH with the reductase is thioredoxin, a 12-

In turn, reduced thioredoxin is regenerated by electron flow from NADPH. This reaction is catalyzed by thioredoxin reductase, a flavoprotein. Electrons flow from NADPH to bound FAD of the reductase, to the disulfide of oxidized thioredoxin, and then to ribonucleotide reductase and, finally, to the ribose unit:

Thymidylate Is Formed by the Methylation of Deoxyuridylate
The preceding reactions have generated deoxyribonucleotides, including deoxyuridine diphosphate (dUDP). However, uracil-

Tetrahydrofolate is regenerated from the dihydrofolate produced in the synthesis of thymidylate. This regeneration is accomplished by dihydrofolate reductase with the use of NADPH as the reductant:

 CLINICAL INSIGHT
CLINICAL INSIGHTSeveral Valuable Anticancer Drugs Block the Synthesis of Thymidylate
Rapidly dividing cells require an abundant supply of thymidylate, the only nucleotide specific to DNA, for DNA synthesis. The vulnerability of these cells to the inhibition of TMP synthesis has been exploited in cancer chemotherapy. Thymidylate synthase and dihydrofolate reductase are choice targets of chemotherapy (Figure 32.9).

Fluorouracil, an anticancer drug, is converted in vivo into fluorodeoxyuridylate (F-
The synthesis of TMP can also be blocked by inhibiting the regeneration of tetrahydrofolate. Analogs of dihydrofolate, such as aminopterin and methotrexate (amethopterin), are potent competitive inhibitors of dihydrofolate reductase:

Methotrexate is a valuable drug in the treatment of many rapidly growing tumors, such as those in acute leukemia and choriocarcinoma, a cancer derived from placental cells. However, methotrexate kills rapidly replicating cells whether they are malignant or not. Stem cells in bone marrow, epithelial cells of the intestinal tract, and hair follicles are vulnerable to the action of this folate antagonist, accounting for its toxic side effects, which include weakening of the immune system, nausea, and hair loss.

Folate analogs such as trimethoprim have potent antibacterial and antiprotozoal activity. Trimethoprim binds 105-fold less tightly to mammalian dihydrofolate reductase than it does to reductases of susceptible microorganisms. Small differences in the activesite clefts of these enzymes account for trimethoprim’s highly selective antimicrobial action. The combination of trimethoprim and sulfamethoxazole (an inhibitor of folate synthesis) is widely used to treat infections such as bronchitis, traveler’s diarrhea, and urinary tract infections.