
33.4 Eukaryotic DNA Is Associated with Specific Proteins
✓ 2 Describe how DNA is packaged to fit inside the cell.
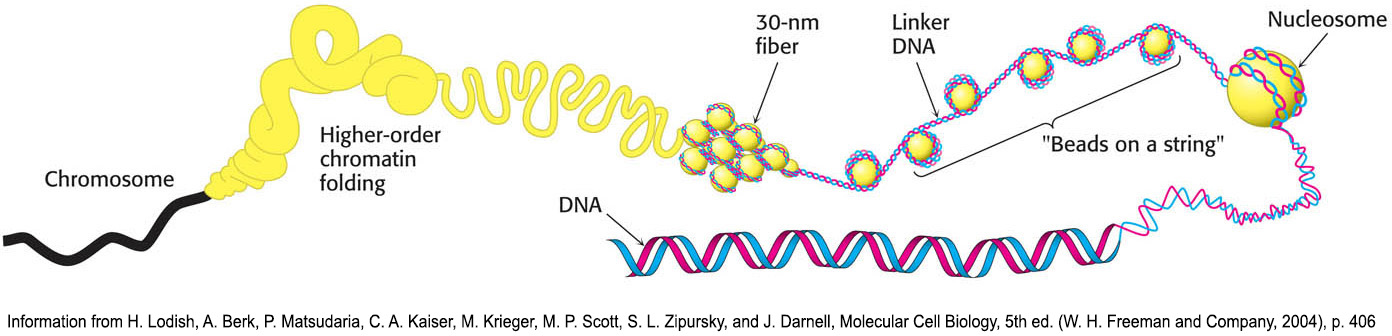
There are about 3.6 m of DNA in each human cell, which are packaged into 46 chromosomes, all of which are in a nucleus that has a diameter of about 5 μm. Clearly, the length of DNA and the size of the nucleus present a packaging problem. Supercoiling contributes to the compaction of DNA. However, further compaction is necessary while, at the same time, maintaining the accessibility of base-
Nucleosomes Are Complexes of DNA and Histones
Eukaryotic DNA is tightly bound to a group of small basic proteins called histones. In fact, histones constitute half the mass of a eukaryotic chromosome. The entire complex of a cell’s DNA and associated protein is called chromatin. Chromatin is essentially isolated chromosomes, although the exact relation between the two remains to be determined. Five major histones are present in chromatin: four histones, called H2A, H2B, H3, and H4, associate with one another; the other histone is called H1. Histones have strikingly basic properties because a quarter of the residues in each histone are either arginine or lysine.
Chromatin is made up of repeating units, each containing 200 bp of DNA and two copies each of H2A, H2B, H3, and H4, called the histone octamer. These repeating units are known as nucleosomes. When chromatin is stretched out so that the DNA and associated nucleosomes can be seen by an electron microscope, it has the appearance of beads on a string; each bead has a diameter of approximately 100 Å (Figure 33.24). Partial digestion of chromatin with DNAse, an enzyme that hydrolytically cleaves the phosphodiester backbone of the DNA, yields the isolated beads. These particles consist of fragments of DNA ≈200 bp in length bound to the eight histones. More extensive digestion yields a DNA fragment of 145 bp bound to the histone octamer. This smaller complex of the histone octamer and the 145-
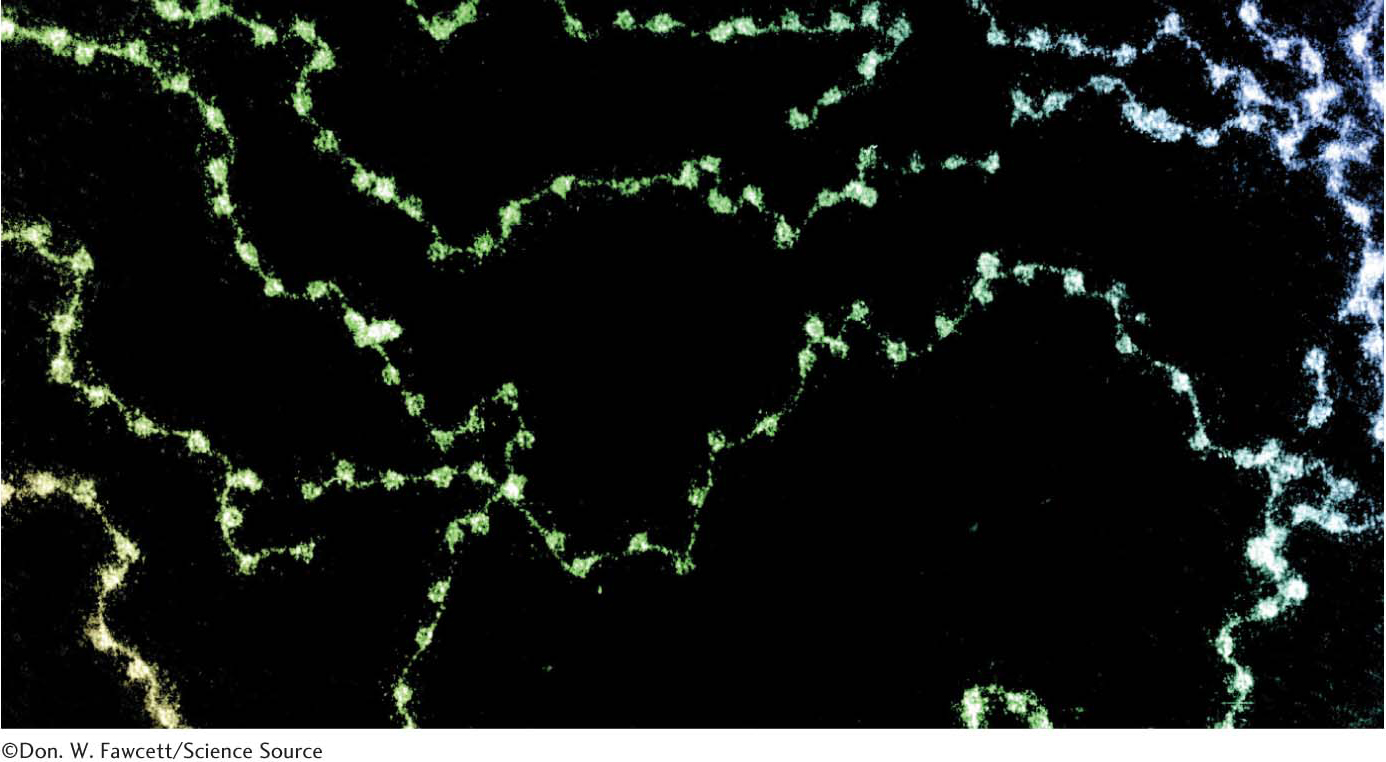
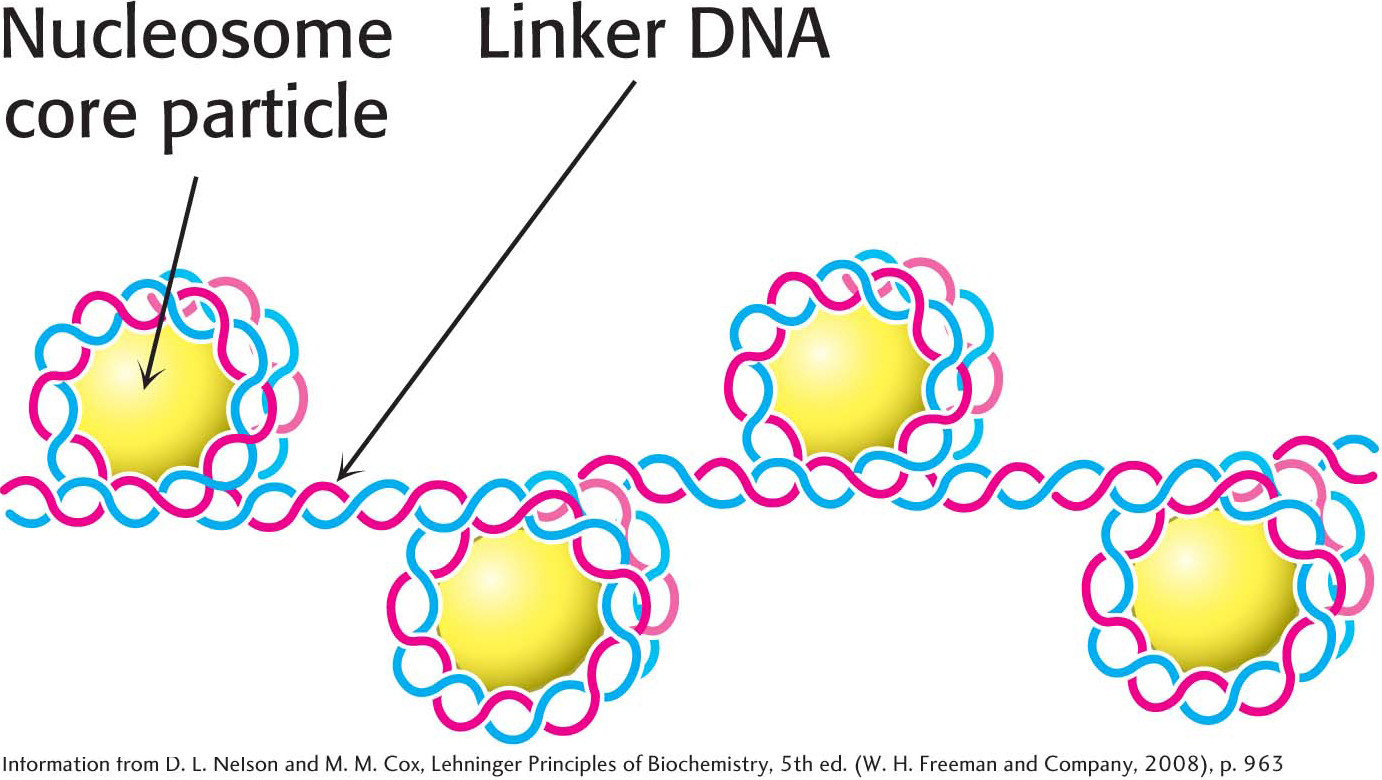
Eukaryotic DNA Is Wrapped Around Histones to Form Nucleosomes
The eight histones in the core particle are arranged into an (H3)2(H4)2 tetramer and a pair of H2A–
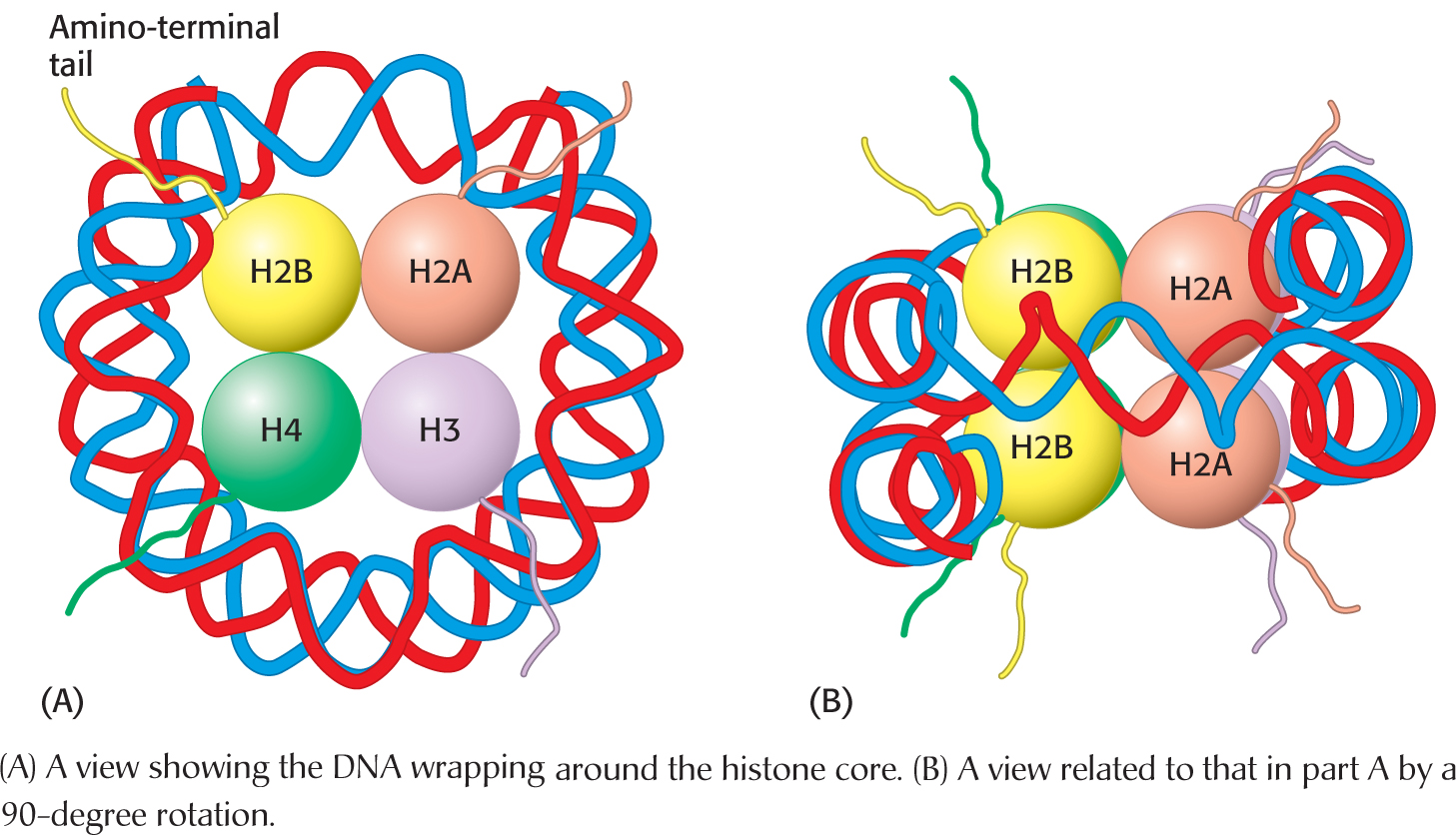
The DNA forms a left-
QUICK QUIZ 2
Why are supercoiling and nucleosome formation important for the structure of DNA in a cell?
Both supercoiling and nucleosome formation help to compact the DNA.
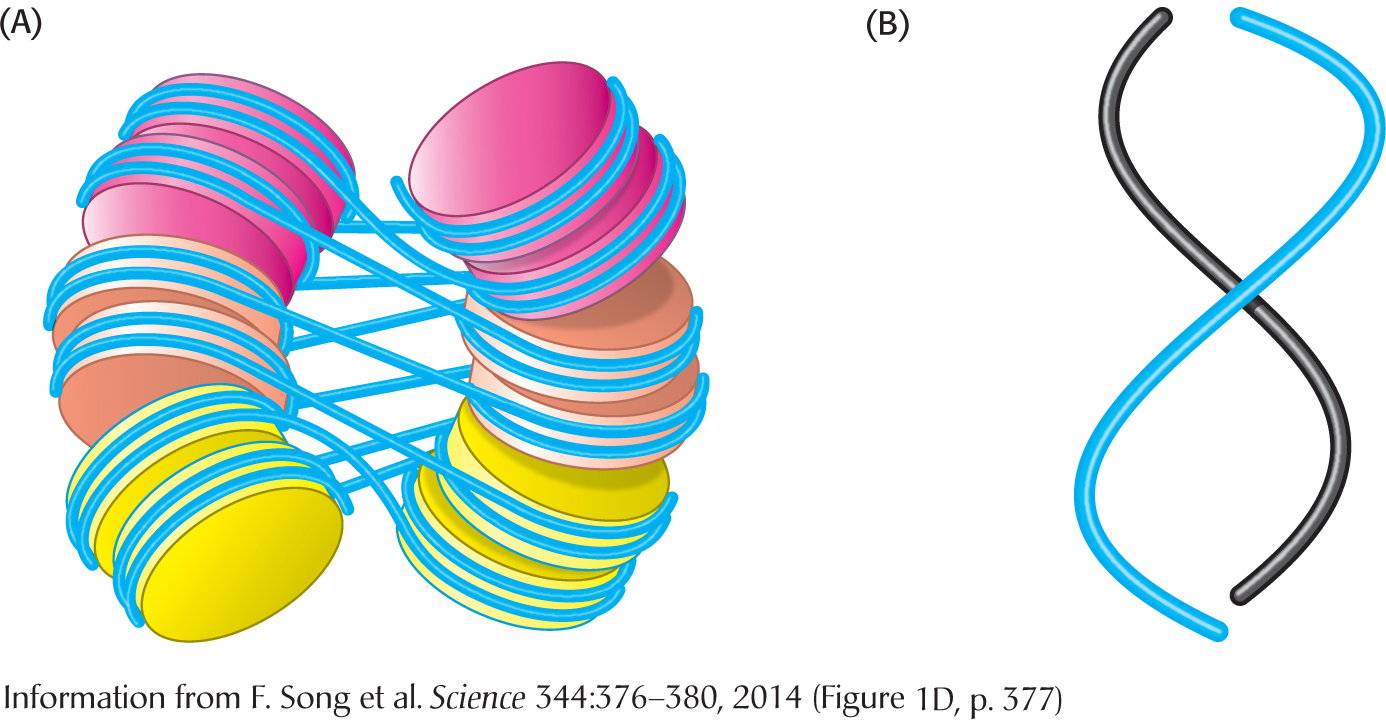
The winding of DNA around the nucleosome core contributes to DNA’s packing by decreasing its linear extent. An extended 200-
 CLINICAL INSIGHT
CLINICAL INSIGHTDamaging DNA Can Inhibit Cancer-Cell Growth
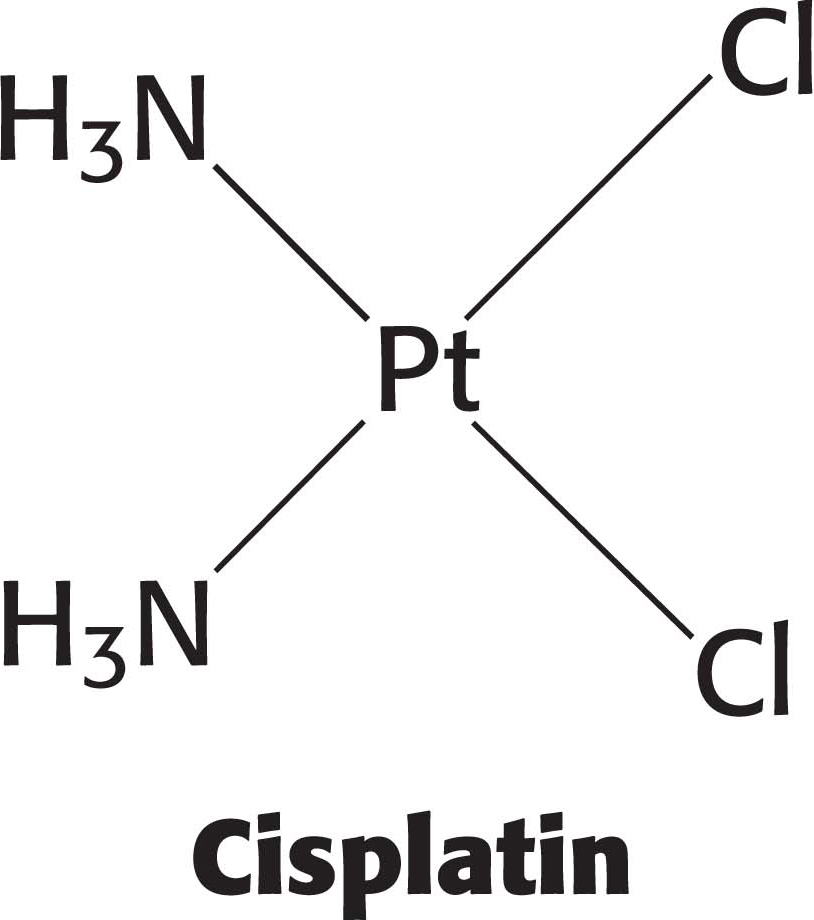
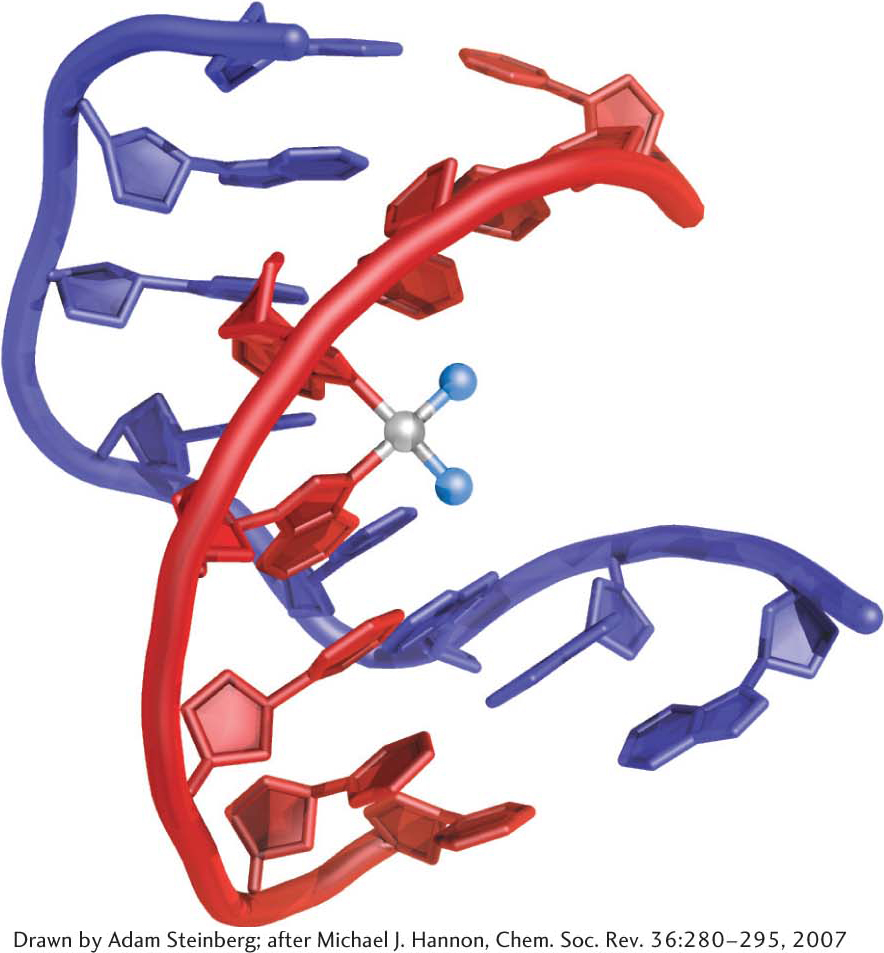
Some cancer chemotherapy drugs work by modifying the structure of DNA, thereby inhibiting replication and transcription. Because cancer cells grow and divide faster than most nonmalignant cells, chemotherapy drugs have a more pronounced effect on cancer cells. An example of a chemotherapy drug is cisplatin, which is used to treat a variety of cancers, including testicular cancer and ovarian cancer. When cisplatin reacts with DNA, the chloride ligands are displaced by purine nitrogen atoms, most commonly in guanine, on two adjacent bases on the same strand. The formation of these bonds causes a severe kink in the structure of the DNA (Figure 33.29). This structural disruption prevents replication and transcription. Moreover, research results suggest that certain nuclear proteins bind to the cisplatin-