
36.3 The lac Operon Illustrates the Control of Bacterial Gene Expression
✓ 2 Describe how transcription is controlled in bacteria.
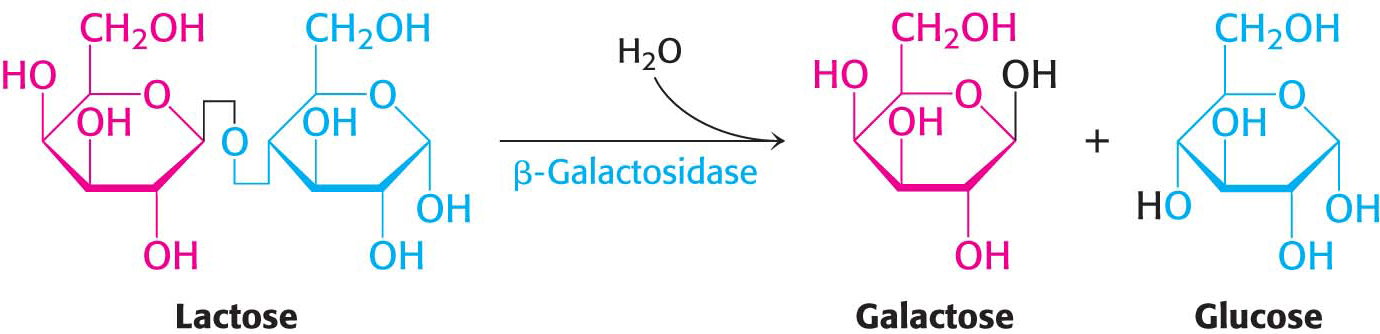
Let us now examine how transcription in bacteria responds to environmental information. An especially clear example is the regulation of genes for the enzymes that catabolize the disaccharide lactose. Bacteria such as E. coli usually rely on glucose as their source of carbon and energy. However, when glucose is scarce, E. coli can use lactose as its carbon source even though this disaccharide does not lie on any major metabolic pathways. An essential enzyme in the metabolism of lactose is β-galactosidase, which hydrolyzes lactose into galactose and glucose. These products are then metabolized by pathways discussed in Chapter 16.
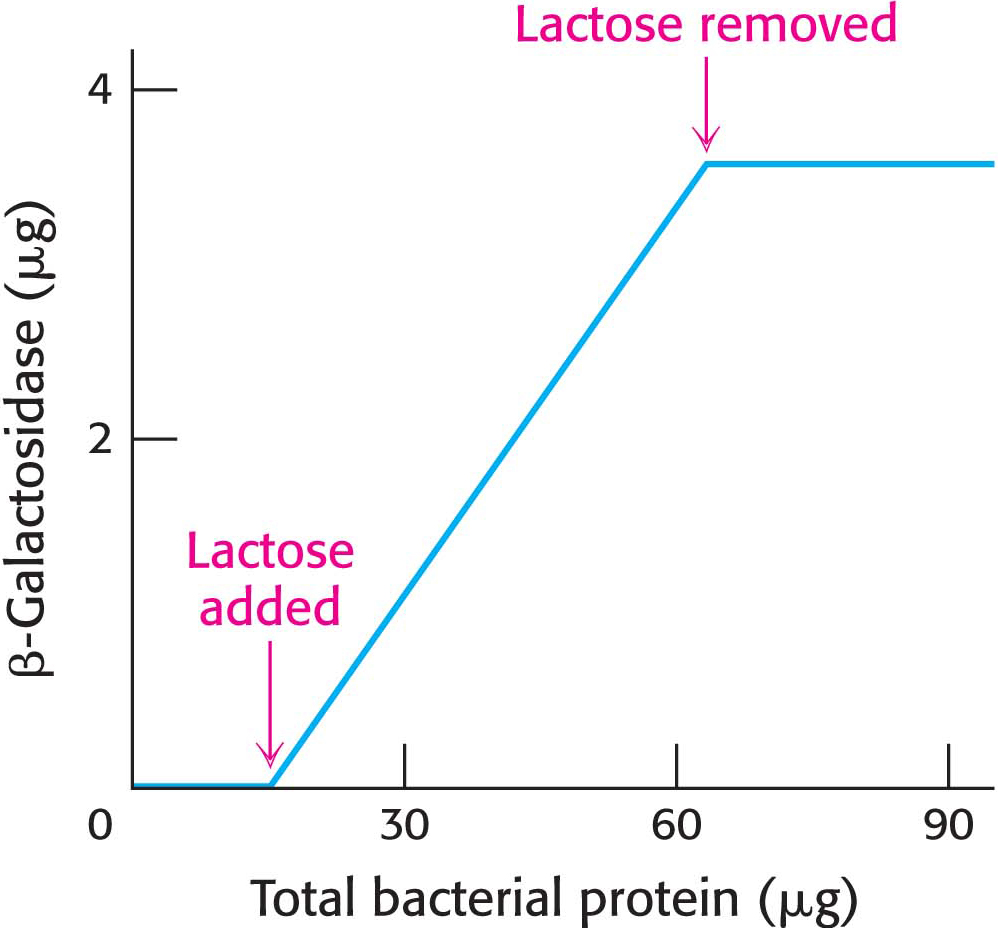
An E. coli cell growing on a carbon source such as glucose or glycerol contains fewer than 10 molecules of β-galactosidase. In contrast, the same cell will contain several thousand molecules of the enzyme when grown on lactose (Figure 36.16).
Interestingly, two other proteins are synthesized whenever β-galactosidase is synthesized—
An Operon Consists of Regulatory Elements and Protein-Encoding Genes
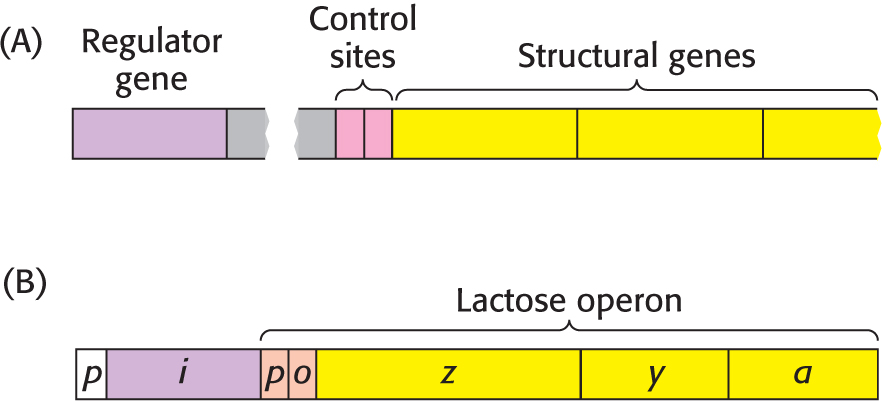
The parallel regulation of β-galactosidase, the permease, and the transacetylase suggests that the expression of genes encoding these enzymes is controlled by a single mechanism (Figure 36.17). The DNA components of the regulatory system are a regulator gene, an operator site, and a set of structural genes that, in the galactosidase example, encode the three enzymes. The regulator gene encodes a repressor protein that binds to the operator site. As the name suggests, binding of the repressor to the operator represses transcription of the structural genes. The operator and its associated structural genes constitute the operon.
For the lactose (lac) operon, the gene encoding the repressor is designated i, the operator site is o, and the structural genes for β-galactosidase, the permease, and the transacetylase are called z, y, and a, respectively. The operon also contains a promoter site (denoted by p), which directs the RNA polymerase to the correct transcription-
How does the lac repressor inhibit the expression of the lac operon? In the absence of lactose, the repressor binds very tightly and rapidly to the operator, blocking the bound RNA polymerase from using the DNA as a template. The biochemical rationale is that, in the absence of lactose, there is no need to transcribe the genes that encode enzymes required for lactose degradation.
Ligand Binding Can Induce Structural Changes in Regulatory Proteins
How is the repressor removed from the operator? This question is tantamount to asking what signal triggers the expression of the lac operon. A signal molecule called an inducer binds to the lac repressor, causing a structural change in the repressor that greatly reduces the affinity of the repressor for the operator DNA. The repressor–
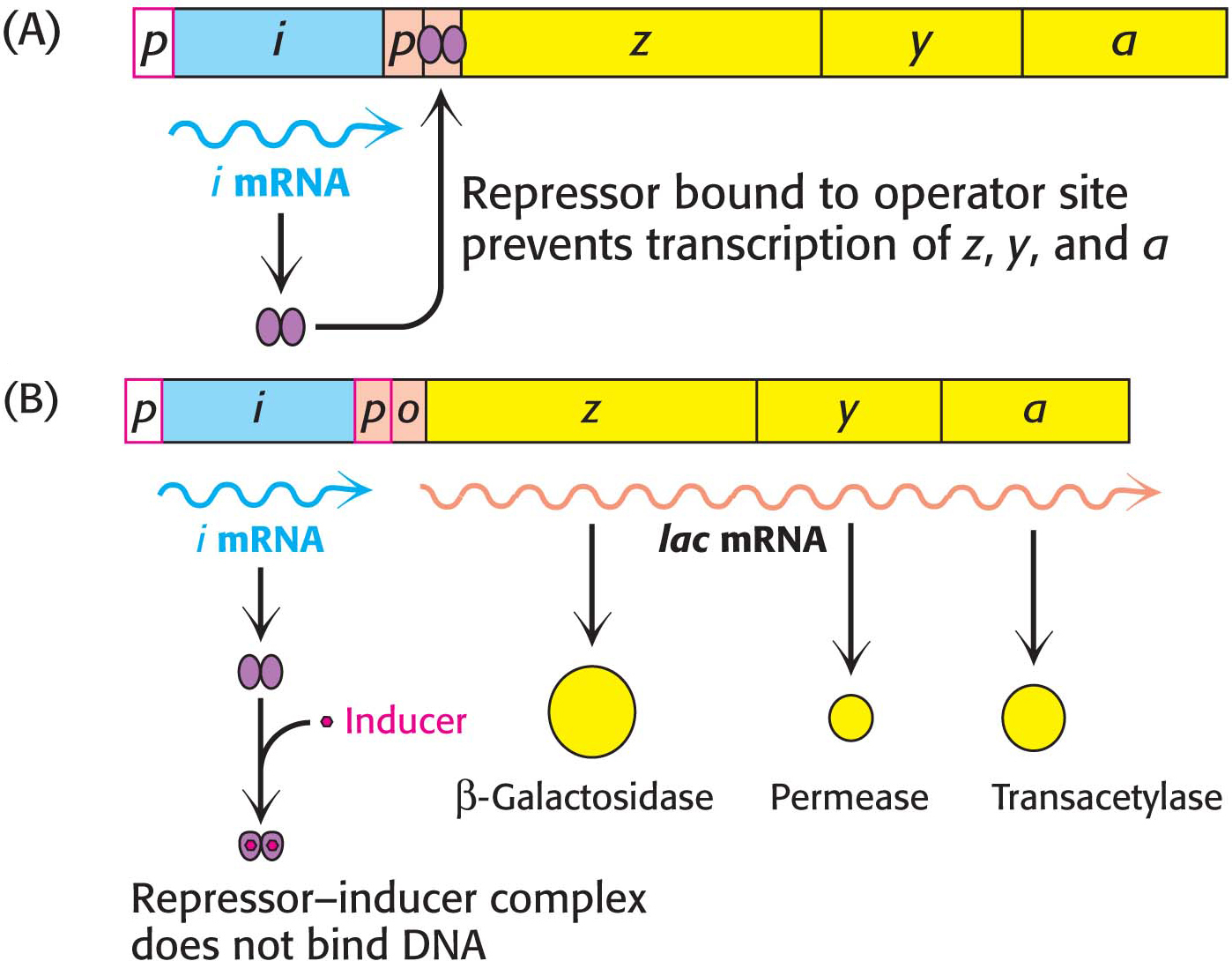
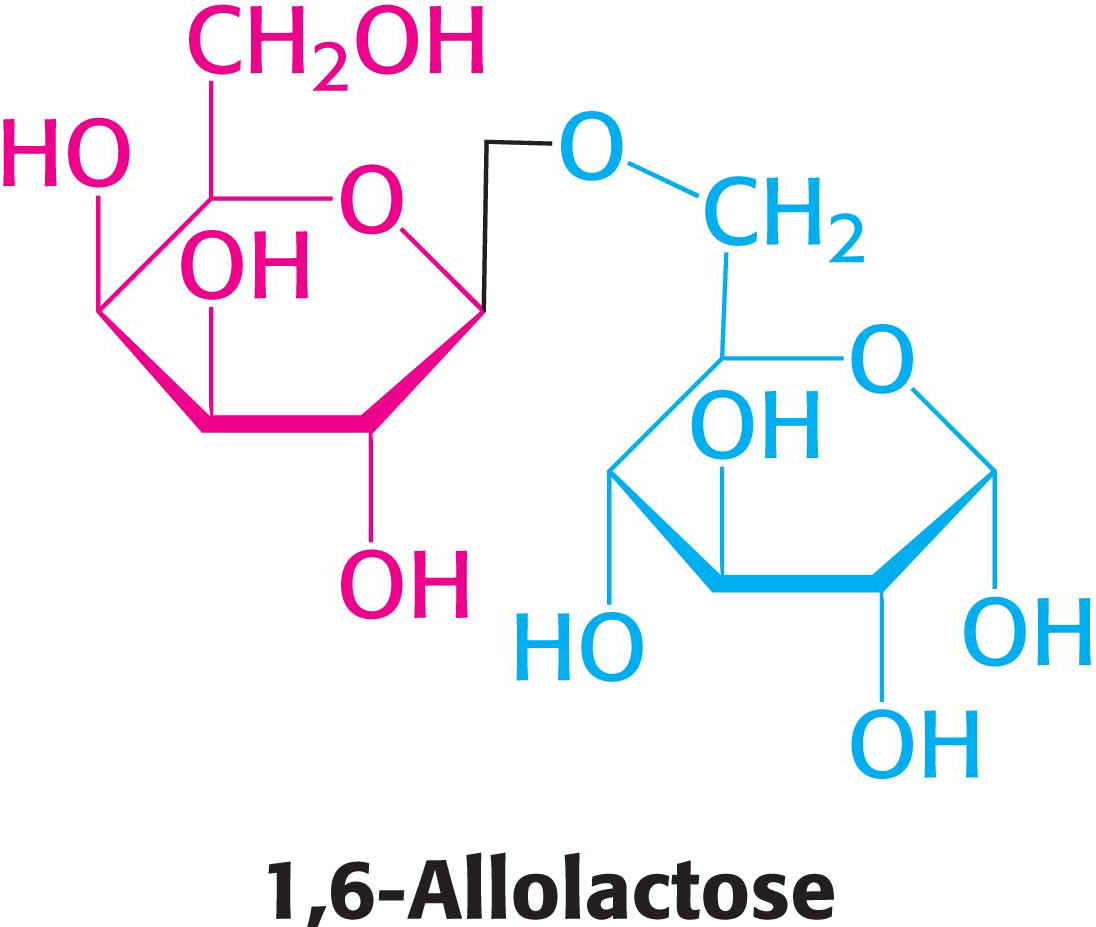
What is the inducer? Intuitively, we would expect it to be lactose itself. Interestingly, however, it is not lactose that announces its own presence; rather, it is allolactose, a combination of galactose and glucose with an α-1,6 rather than an α-1,4 linkage, as in lactose. Allolactose is a side product of the β-galactosidase reaction produced at low levels by the few molecules of β-galactosidase that are present before induction. Many other gene-
Transcription Can Be Stimulated by Proteins That Contact RNA Polymerase
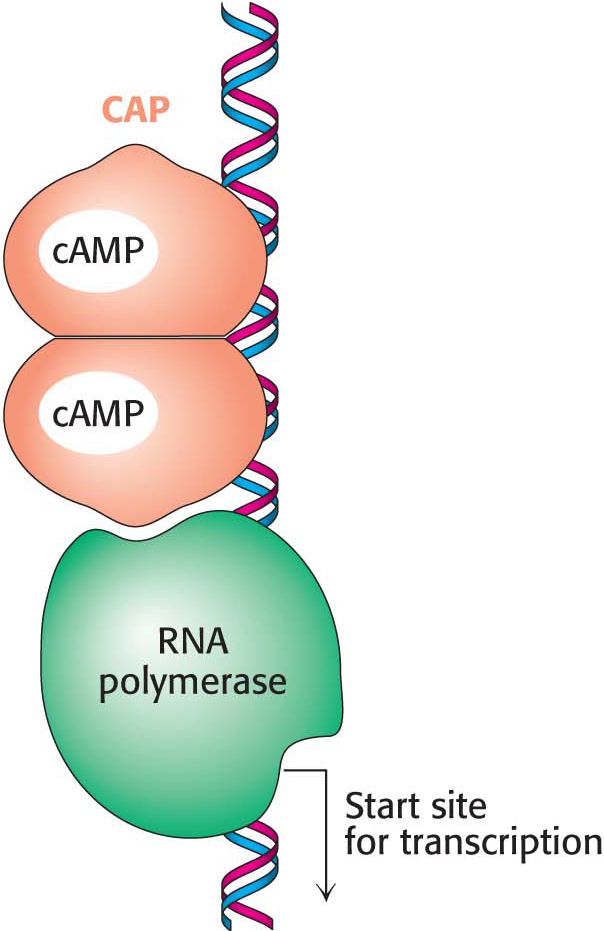
The lac repressor functions by inhibiting transcription until lactose is present. There are also sequence-
Within the lac operon, the CAP–
QUICK QUIZ 2
Describe how allolactose and cAMP combine to regulate the lac operon.
Allolactose binds to the repressor protein, preventing the repressor from binding to the operator and allowing the polymerase access to the gene so that transcription can take place. Cyclic AMP binds to CAP. This complex binds to the lac operon upstream of the promoter and stimulates the binding of polymerase to the promoter.
How is the level of cAMP controlled in bacteria? Enzyme IIA (EIIA) is phosphorylated at the expense of the glycolytic intermediate phosphoenolpyruvate. Phosphorylated EIIA then transfers a phosphate to a glucose molecule, generating glucose 6-


 CLINICAL AND BIOLOGICAL INSIGHT
CLINICAL AND BIOLOGICAL INSIGHTMany Bacterial Cells Release Chemical Signals that Regulate Gene Expression in Other Cells
Bacterial cells have been traditionally viewed as solitary single cells. However, it is becoming increasingly clear that, in many circumstances, bacterial cells live in complex communities, interacting with other cells of their own species and of different species. These social interactions change the patterns of gene expression within the cells.
A prominent type of interaction is called quorum sensing. This phenomenon was discovered in Vibrio fischeri, a species of bacterium that can live inside a specialized light organ in the bobtail squid (Figure 36.20). The bobtail squid inhabits shallow waters and hunts at night. On moonlit nights, the squid would be visible to predators prowling below it. The squid adjusts the light emitted by the light organ to match the background, thus becoming less visible to predators. In this symbiotic relation, the bacteria produce the enzyme luciferase, which generates bioluminescence, thereby providing protection for the squid. In return, the bacteria have a protected place to live and reproduce. Interestingly, when these bacteria are grown in culture at low density, they are not bioluminescent. However, when the cell density reaches a critical level, the gene for luciferase is expressed and the cells bioluminesce. How do these cells sense the density of their population?
Cells of V. fischeri release an autoinducer into their environment and other V. fischeri cells take up the chemical. After the inducer concentration inside the cell has increased to an appropriate level, the inducer binds to a regulatory protein. The complex of inducer–
Quorum sensing appears to play a major role in the formation of bacterial communities with particular species compositions. Many species of bacteria can be found in specialized structures termed biofilms that can form on surfaces. Some genes controlled by quorum-
Some Messenger RNAs Directly Sense Metabolite Concentrations
A newly discovered control mechanism for the regulation of gene expression depends on the remarkable ability of some mRNA molecules to form special secondary structures, some of which are capable of directly binding small molecules. These structures are termed riboswitches. Consider a riboswitch-