
37.2 RNA Polymerase II Requires Complex Regulation
✓ 3 Describe how transcription is regulated in eukaryotes.

The elaborate regulation of RNA polymerase II accounts for cell differentiation and development in higher organisms. Consequently, we will focus our attention on this vital enzyme. Promoters for RNA polymerase II, like those for bacterial polymerases, are generally located on the 5′ side of the start site for transcription. The most commonly recognized cis-
The TATA box is often paired with an initiator element (Inr), a sequence found at the transcriptional start site located at ∼ +1. This sequence defines the start site because the other promoter elements are at variable distances from that site. Its presence increases transcriptional activity.
A third element, the downstream core promoter element (DPE), is commonly found in conjunction with the Inr in transcripts that lack the TATA box. In contrast with the TATA box, the DPE is found downstream of the start site, at ∼ +30.
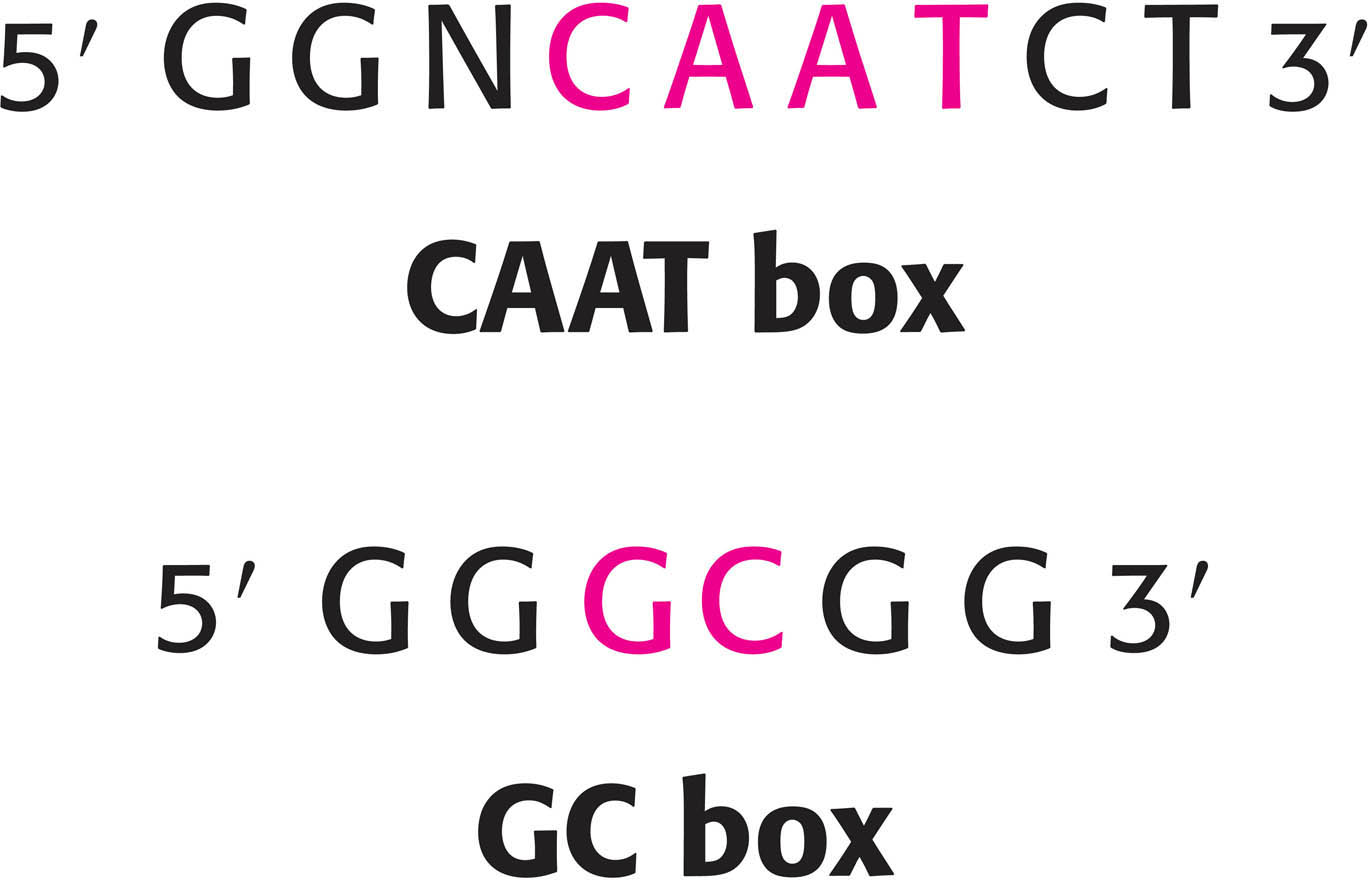
Additional regulatory sequences are located between −40 and −150. Many promoters contain a CAAT box, and some contain a GC box (Figure 37.5). Constitutive genes (genes that are continuously expressed rather than regulated) tend to have GC boxes in their promoters. The positions of these upstream sequences vary from one promoter to another, in contrast with the location of the −35 region in bacteria. Another difference is that the CAAT box and the GC box can be effective when present on the template (antisense) strand, unlike the −35 region, which must be present on the coding (sense) strand. These differences between bacteria and eukaryotes correspond to fundamentally different mechanisms for the recognition of cis-
The Transcription Factor IID Protein Complex Initiates the Assembly of the Active Transcription Complex
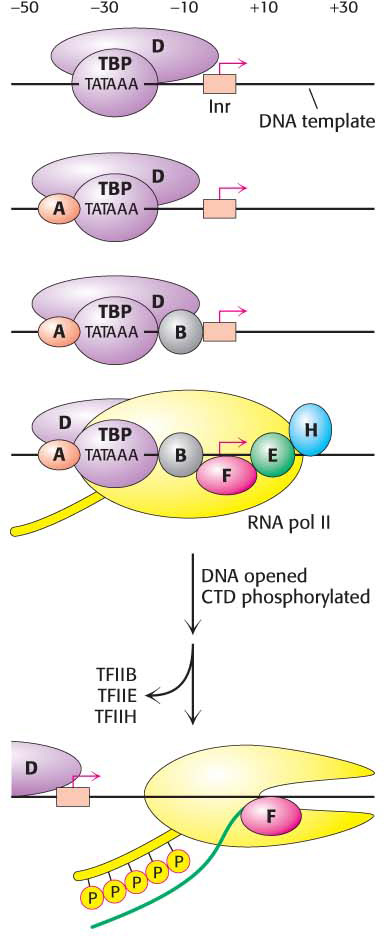
Cis-
In TATA-
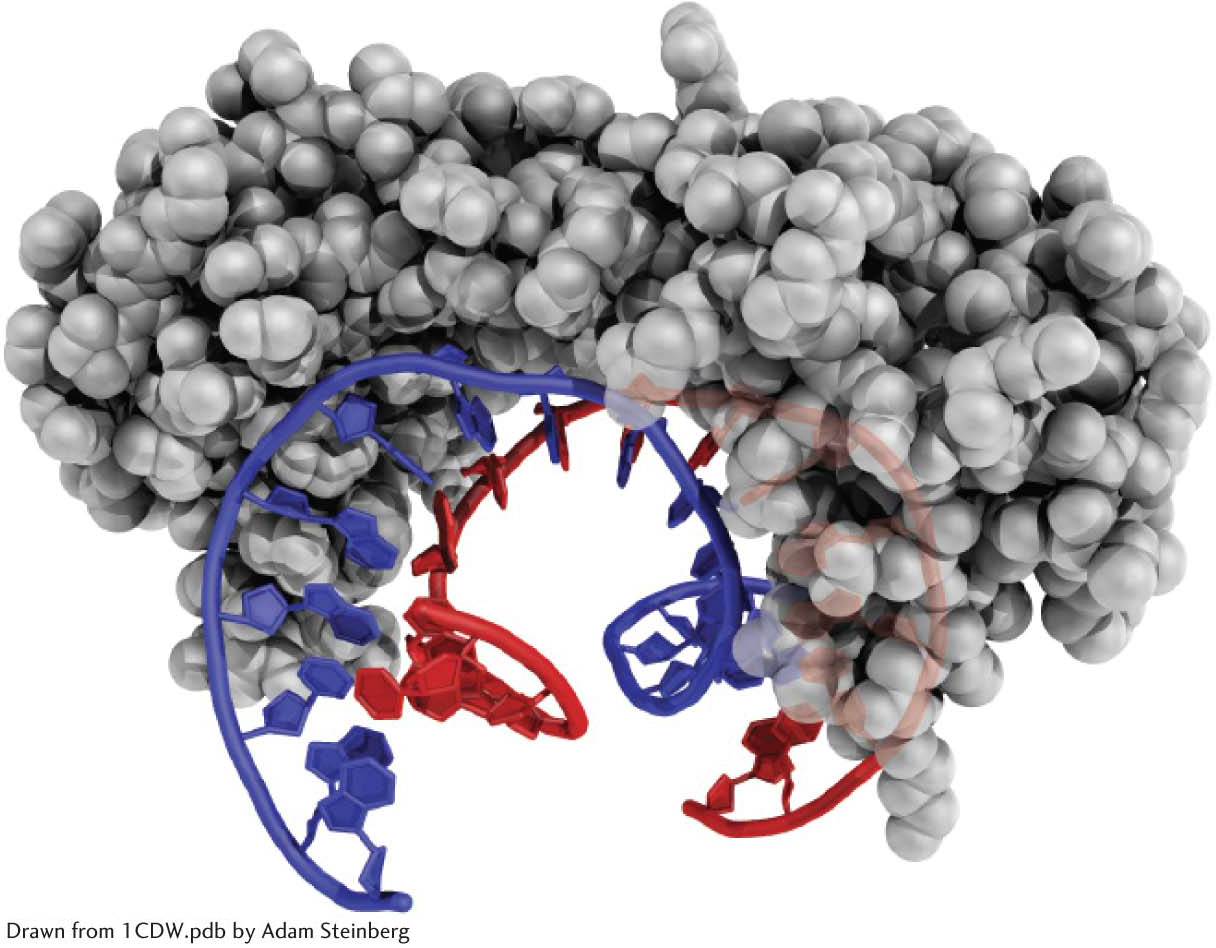
TBP bound to the TATA box nucleates the formation of the preinitiation complex (PIC) (Figure 37.6). The surface of the TBP saddle provides docking sites for the binding of other components. TFIIA and TFIIB bind next, stabilizing the binding of TBP to the DNA. With the arrival of TFIIB, the complex recruits RNA polymerase, which is escorted to the promoter site by TFIIE. Binding by TFIIH completes the formation of the PIC.
TFIIH has two essential catalytic activities. First, it is an ATP-
Enhancer Sequences Can Stimulate Transcription at Start Sites Thousands of Bases Away
The activities of many promoters in higher eukaryotes are greatly increased by another type of cis-
QUICK QUIZ 1
Differentiate between a promoter and an enhancer.
A promoter is a DNA sequence that attracts the polymerase to the start site for transcription. Promoters are usually located just upstream of the gene. An enhancer is a DNA sequence that has no promoter activity itself but greatly enhances the activity of an associated promoter. Enhancers can exert their effects over a distance of several thousand base pairs.
 CLINICAL INSIGHT
CLINICAL INSIGHTInappropriate Enhancer Use May Cause Cancer
Enhancer sequences are important in establishing the tissue specificity of gene expression because a particular enhancer is effective only in certain cells. For example, the immunoglobulin enhancer functions in B lymphocytes but not elsewhere. Cancer can result if the relation between genes and enhancers is disrupted. In Burkitt lymphoma and B-
Multiple Transcription Factors Interact with Eukaryotic Promoters and Enhancers
The basal transcription complex described above initiates transcription at a low frequency. Additional transcription factors that bind to other sites are required to achieve a high rate of mRNA synthesis and to selectively stimulate specific genes. Indeed, many transcription factors have been isolated. These transcription factors are often expressed in a tissue-
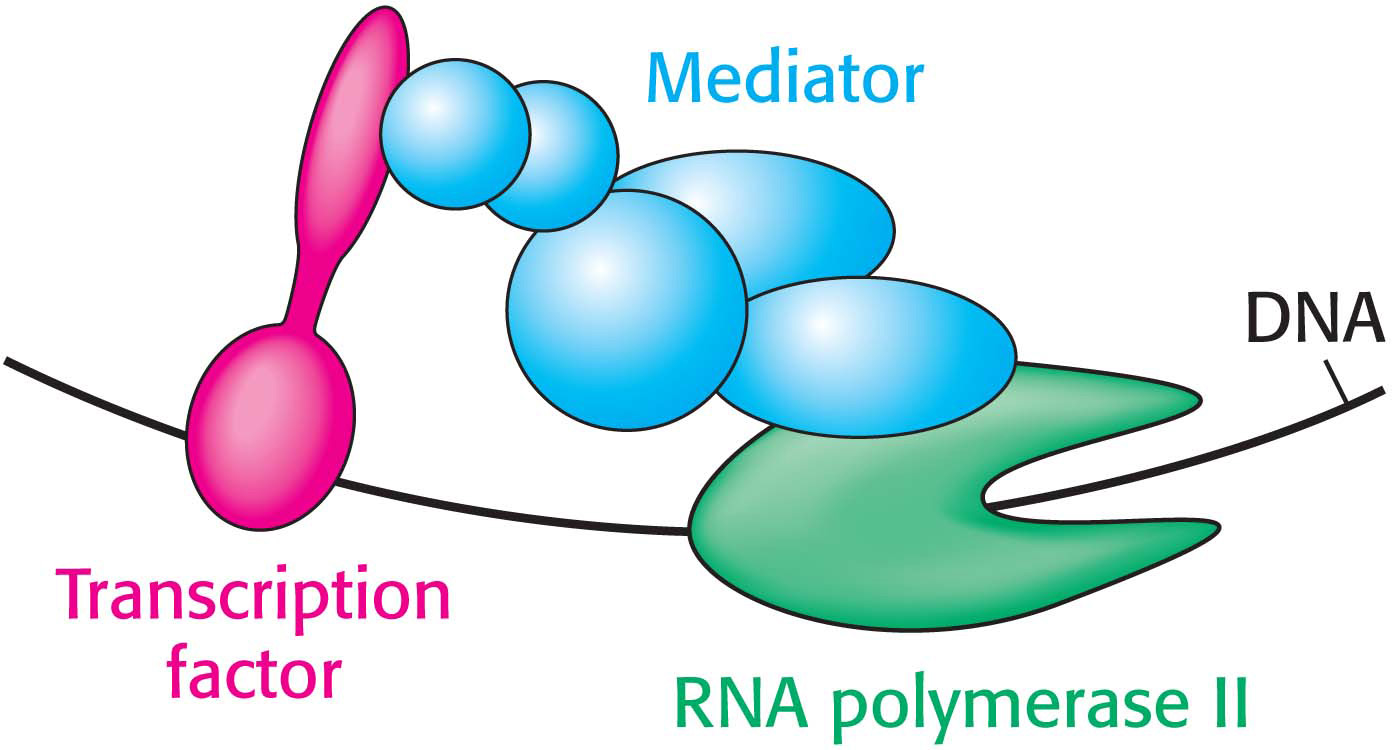
In contrast with those of bacterial transcription, few eukaryotic transcription factors have any effect on transcription on their own. Instead, each factor recruits other proteins to build up large complexes that interact with the transcriptional machinery to activate or repress transcription. These intermediary proteins act as a bridge between the transcription factors and the polymerase. An important target intermediary for many transcription factors is mediator, a huge complex of 25 to 30 subunits with a mass of more than 1-
DID YOU KNOW?
Pluripotent cells are stem cells that can develop into any type of fetal or adult cell. Totipotent stems cells not only can develop into any fetal or adult cell type, but also can develop extraembryonic tissues and thus grow into an entire organism.
Transcription factors and other proteins that bind to regulatory sites on DNA can be regarded as passwords that cooperatively open multiple locks, giving RNA polymerase access to specific genes. A major advantage of this mode of regulation is that a given regulatory protein can have different effects, depending on what other proteins are present in the same cell. This phenomenon, called combinatorial control, is crucial to multicellular organisms that have many different cell types. A comparison between human beings and the simple roundworm Caenorhabditis elegans provides an example of the power of combinatorial control. Human beings have only one-
 CLINICAL INSIGHT
CLINICAL INSIGHTInduced Pluripotent Stem Cells Can Be Generated by Introducing Four Transcription Factors into Differentiated Cells
An important application illustrating the power of transcription factors is the development of induced pluripotent stem (iPS) cells. Pluripotent stem cells, which can be derived from embryos, have the ability to differentiate into many different cell types on appropriate treatment. Recent experiments have identified just four genes, encoding transcription factors, that can induce pluripotency in already-
These iPS cells are powerful new research tools and, potentially, a new class of therapeutic agents. Ideally, a sample of a patient’s fibroblasts could be readily isolated and converted into iPS cells. These iPS cells could then be treated to differentiate into a desired cell type that could be transplanted into the patient, repairing tissue damage. Although the field of iPS-
