37.3 Gene Expression Is Regulated by Hormones
Just as bacteria can adjust their patterns of gene expression in response to chemicals in their environment, eukaryotes have many systems for responding to environmental stimuli. As the first step in the control of gene expression, the initiation of transcription by RNA polymerase II is the focal point for many signal-
Because they are hydrophobic signal molecules, estrogens easily diffuse across cell membranes. When inside a cell, estrogens bind to highly specific receptor proteins. Estrogen receptors are members of a large family of proteins that act as receptors for a wide range of hydrophobic molecules. The steroid receptors are different from the receptors discussed thus far in that they are soluble and located in the cytoplasm or nucleoplasm, rather than being bound to membranes. This receptor family includes receptors for other steroid hormones, such as the androgen testosterone, as well as thyroid hormones, and vitamin A-
Nuclear Hormone Receptors Have Similar Domain Structures
Nuclear hormone receptors bind to specific DNA sites referred to as response elements. In regard to the estrogen receptor, the estrogen response elements (EREs) contain the consensus sequence 5′-AGGTCANNNTGACCT-3′. As expected from the symmetry of this sequence, an estrogen receptor binds to such sites as a dimer.
A comparison of the amino acid sequences of members of the nuclear hormone-
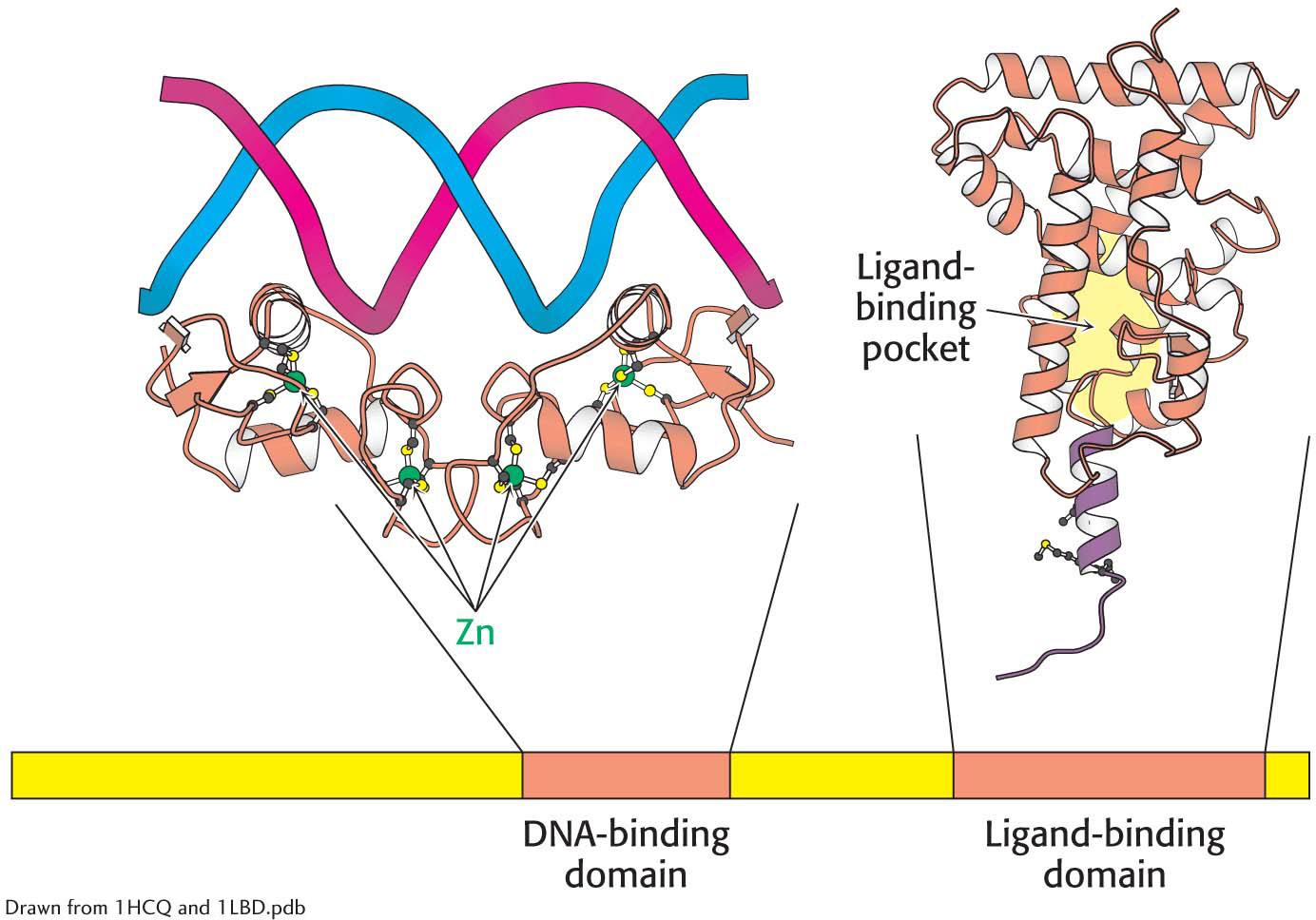

 The structure of two nuclear hormone-
The structure of two nuclear hormone-The second highly conserved region of a nuclear hormone receptor lies near the carboxyl terminus. This area of the receptor forms a hydrophobic pocket that is the ligand-
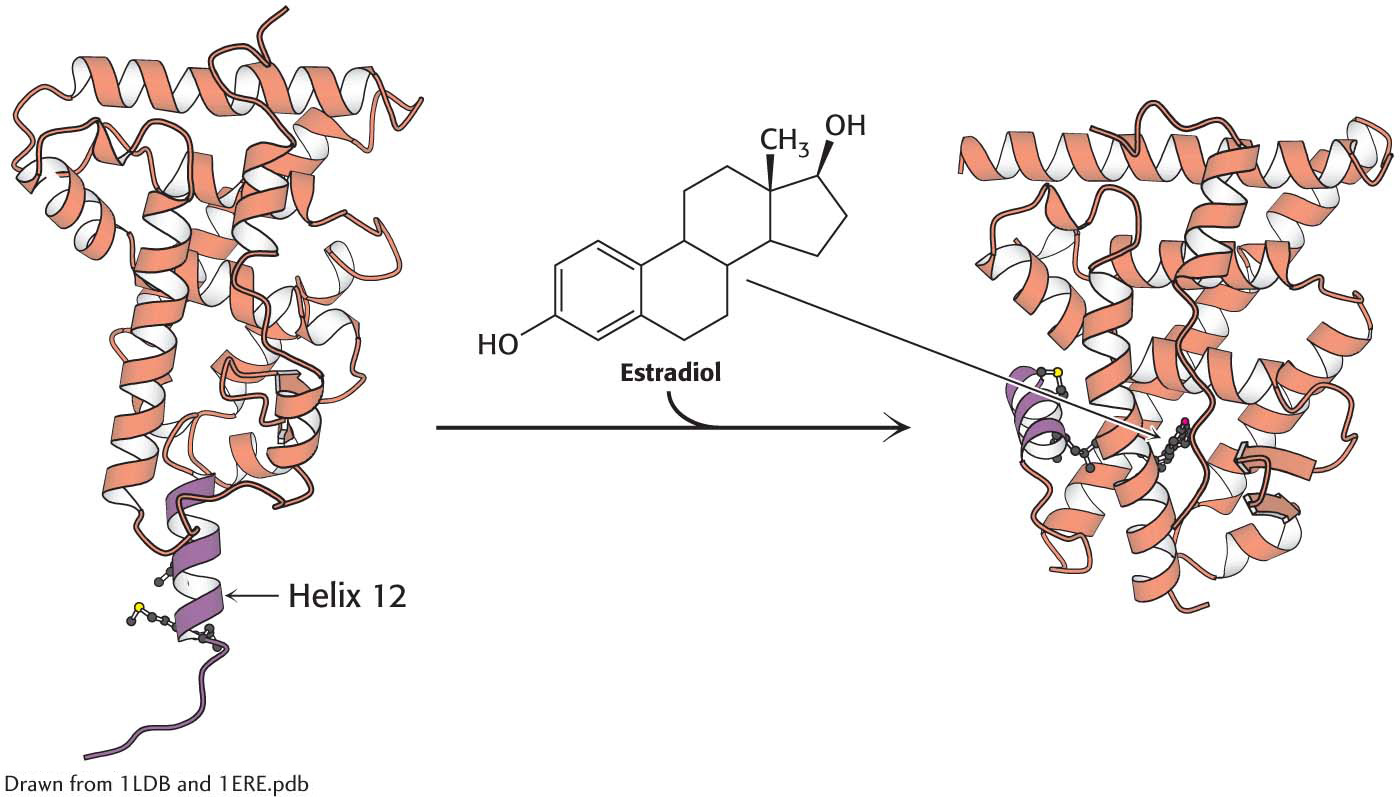

 Ligand binding to nuclear hormone receptor. The ligand binding causes structural alteration in the receptor so that the hormone lies completely surrounded within a pocket in the ligand-
Ligand binding to nuclear hormone receptor. The ligand binding causes structural alteration in the receptor so that the hormone lies completely surrounded within a pocket in the ligand-Nuclear Hormone Receptors Recruit Coactivators and Corepressors
Although the receptor can bind DNA without a ligand, the receptor cannot serve its recruitment function without first binding the ligand. Proteins that bind to the receptor only after it has bound to the steroid are called coactivators. The site for the interaction between the nuclear hormone–
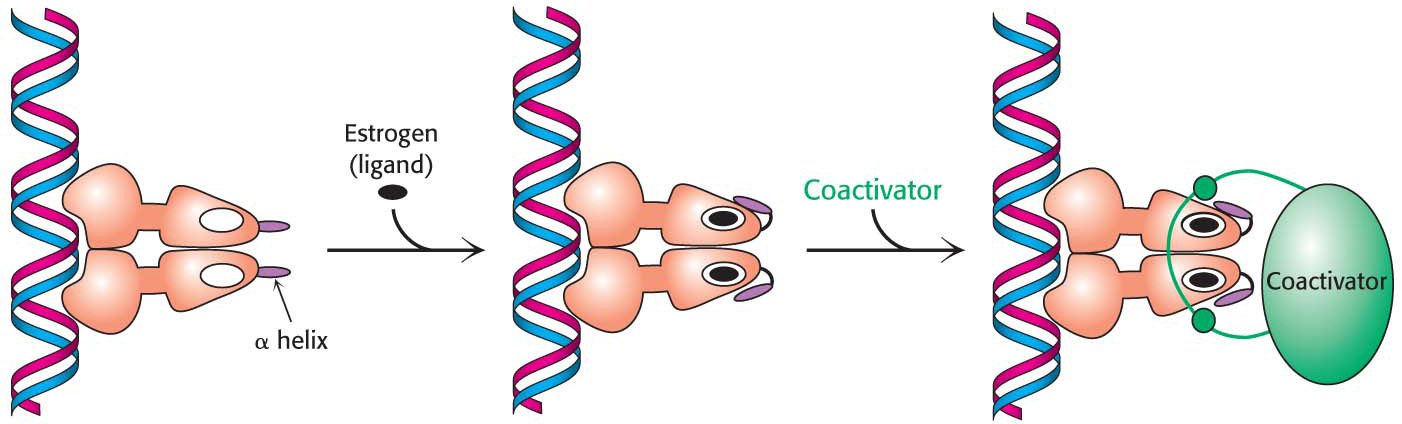
Although the estrogen receptor is inactive in the absence of estrogen, other members of the nuclear hormone-
QUICK QUIZ 2
Outline the mechanism of action of estradiol.
Estradiol diffuses into the cell. It binds to a specific receptor protein. When bound to the hormone, the receptor recruits other proteins called coactivators that stimulate RNA polymerase.
 CLINICAL INSIGHT
CLINICAL INSIGHTSteroid-Hormone Receptors Are Targets for Drugs
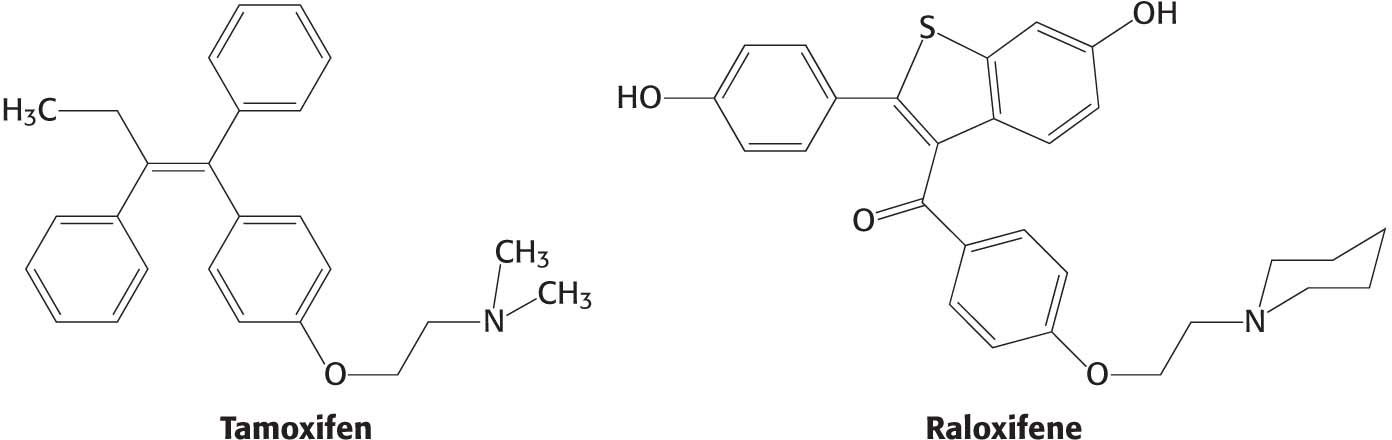
Molecules such as estradiol that bind to a receptor and trigger signaling pathways are called agonists. As discussed in Chapter 29, some athletes take natural and synthetic agonists of the androgen receptor, a member of the nuclear hormone-
Other molecules bind to nuclear hormone receptors but do not effectively trigger signaling pathways. Such compounds are called antagonists and are, in many ways, like competitive inhibitors of enzymes. Some important drugs are antagonists that target the estrogen receptor. For example, tamoxifen and raloxifene are used in the treatment and prevention of breast cancer, because some breast tumors rely on estrogen-