
Amino Acids


The role of amino acids as the building blocks of proteins is readily apparent in health food stores. Indeed, their shelves are stocked with powdered amino acids for use as dietary supplements, enabling body builders to enhance muscle growth. However, amino acids are key biochemicals in their own right. Some amino acids function as signal molecules, such as neurotransmitters, and all amino acids are precursors to other biomolecules, such as hormones, nucleic acids, lipids, and, as just mentioned, proteins. In this chapter, we examine the fundamental chemical properties of amino acids.
Two Different Ways of Depicting Biomolecules Will Be Used
Proteins, as well as biochemicals in general, derive their amazing array of functions from the ability to form three-
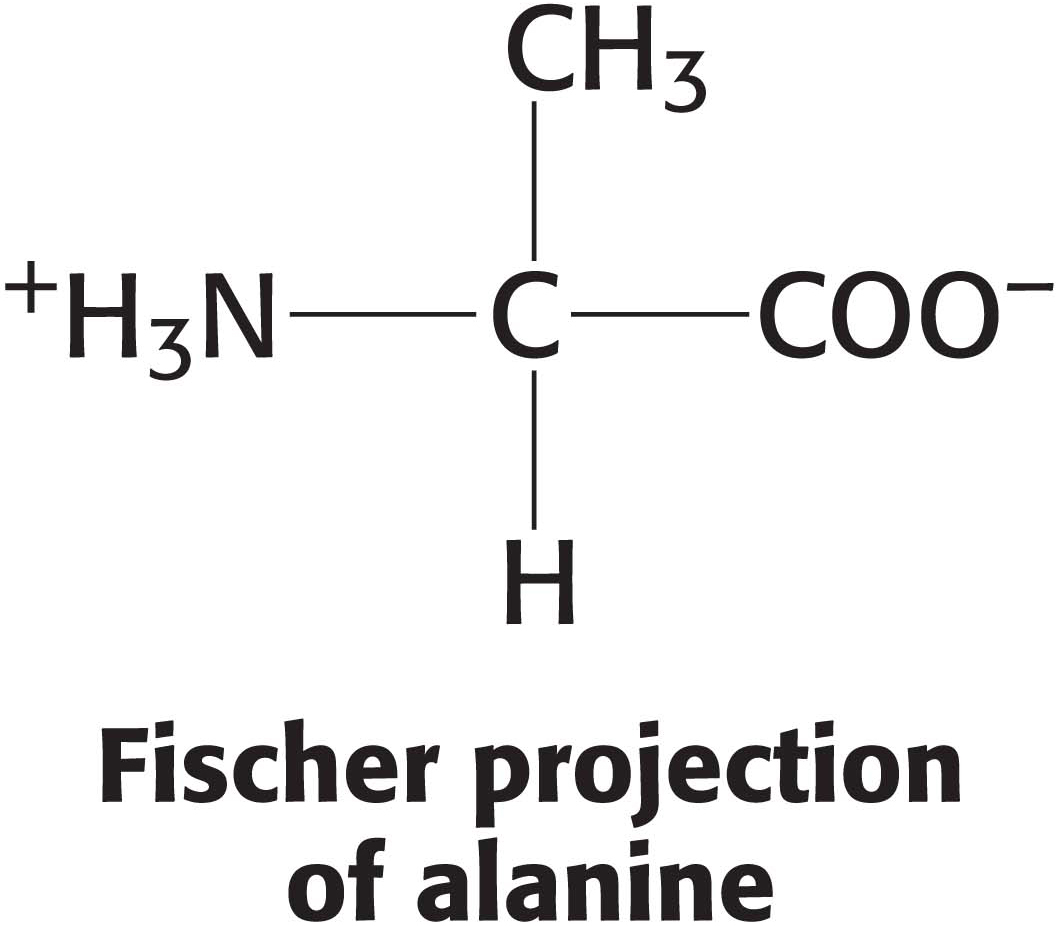
When visualizing the constituent atoms of a molecule (such as carbons and hydrogens) is more important than seeing the shape of the molecule, we use a depiction called a Fischer projection. In a Fischer projection, every atom is identified and the bonds to the central carbon atom are represented by horizontal and vertical lines. By convention, the horizontal bonds are assumed to project out of the page toward the viewer, whereas the vertical bonds are assumed to project behind the page away from the viewer.
When emphasis is on a molecule’s function, visualization of the shape of the molecule is more important. In such instances, stereochemical renderings are used because they convey an immediate sense of the molecule’s structure and, therefore, a hint about its function. Stereochemical renderings also simplify the diagram, thereby making the function of a molecule clearer. Carbon and hydrogen atoms are not explicitly shown unless they are important to the activity of the molecule. In this way, the functional groups are easier to identify.
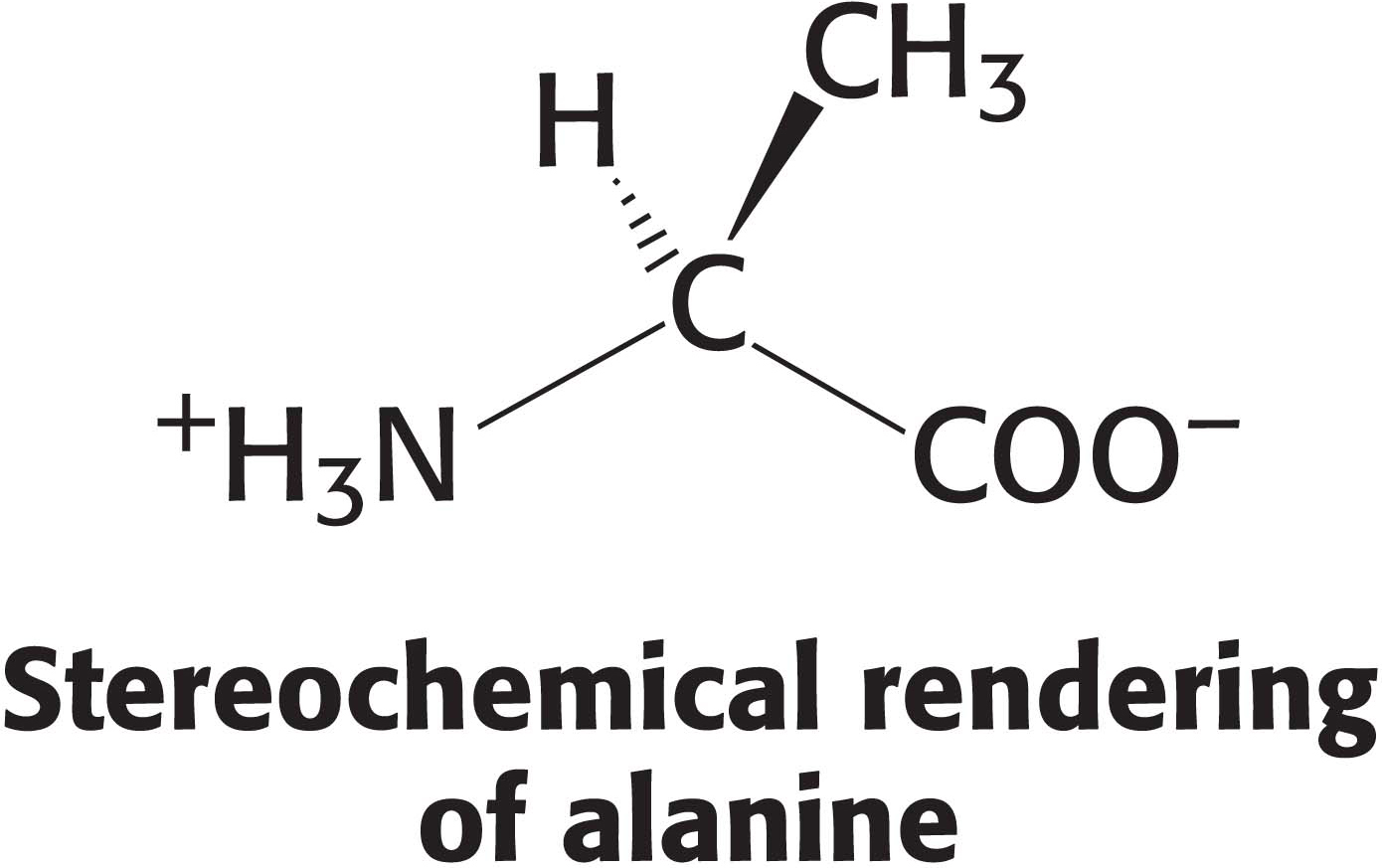
To illustrate the correct stereochemistry of tetrahedral carbon atoms, wedges are used to depict the direction of a bond into or out of the plane of the page. A solid wedge denotes a bond coming out of the plane of the page toward the viewer. A dashed wedge represents a bond going away from the viewer and behind the plane of the page. The remaining two bonds are depicted as straight lines.