3.1 Proteins Are Built from a Repertoire of 20 Amino Acids
✓ 1 Identify the main classes of amino acids.
Amino acids are the building blocks of proteins. An α-amino acid consists of a central carbon atom, called the α carbon, which is bonded to an amino group, a carboxylic acid group, a hydrogen atom, and a side chain, called the R group. Each kind of amino acid has a different R group.
Most Amino Acids Exist in Two Mirror-Image Forms
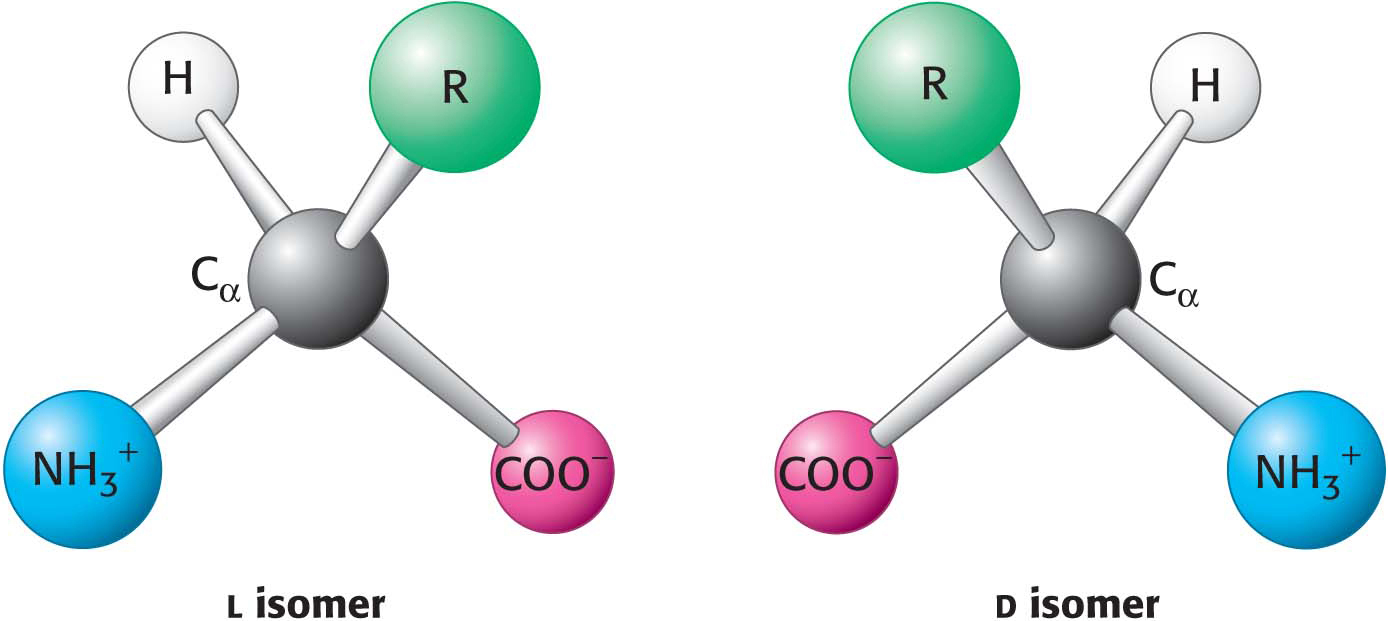
With four different groups connected to the tetrahedral α-carbon atom, α-amino acids are chiral: they may exist in one or the other of two mirror-
Only l amino acids are constituents of proteins. What is the basis for the preference for l amino acids? The answer is not known, but evidence shows that pure l or d amino acids are slightly more soluble than a stable dl crystal. Consequently, if simply by chance, there were a small excess of the l amino acid, this small solubility difference could have been amplified over time so that the l isomer became dominant in solution.
All Amino Acids Have at Least Two Charged Groups
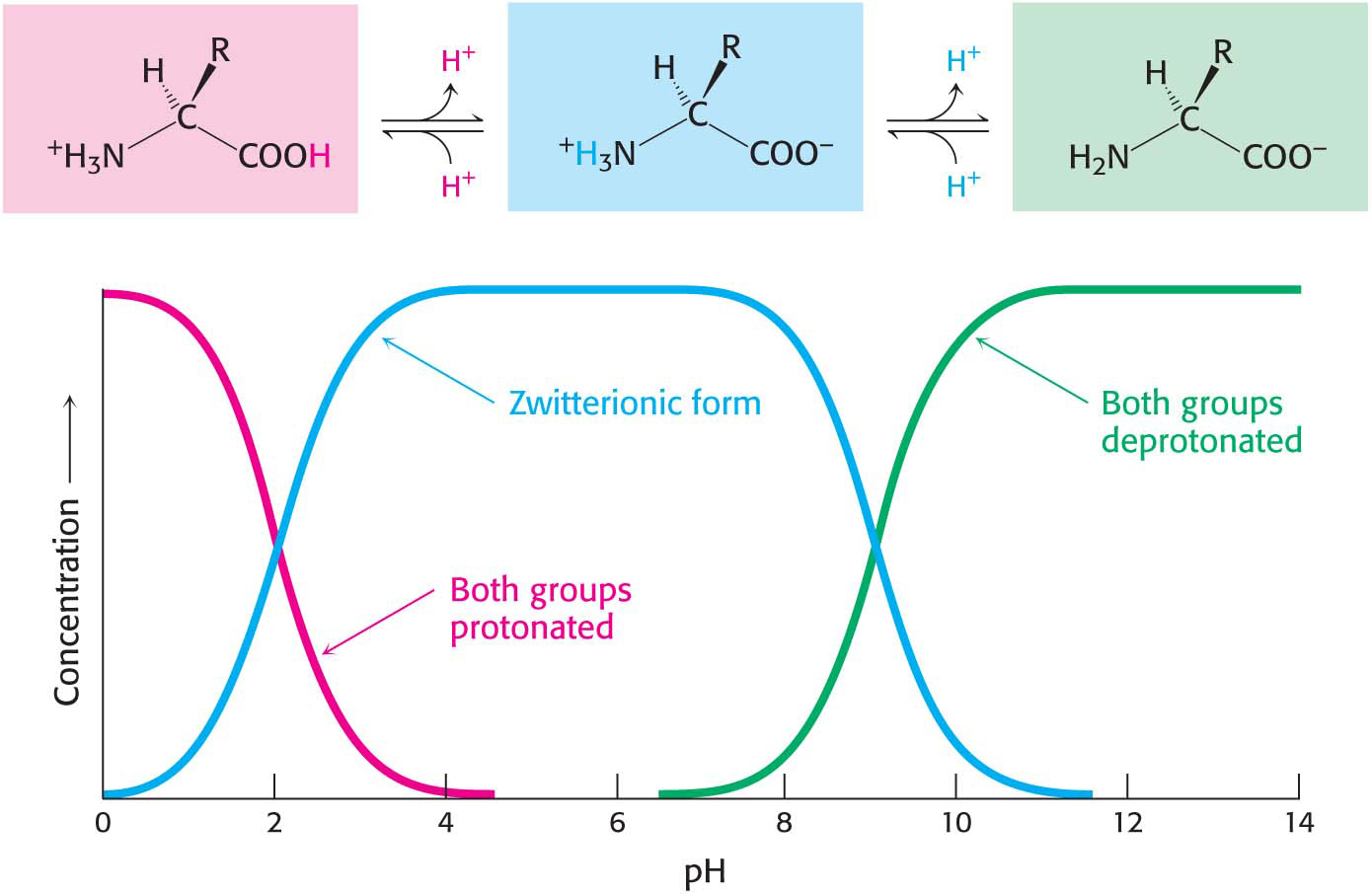
Free amino acids in solution at neutral pH exist predominantly as dipolar ions (also called zwitterions). In the dipolar form, the amino group is protonated (NH3+) and the carboxyl group is deprotonated (COO−). The ionization state of an amino acid varies with pH (Figure 3.2). In acid solution (e.g., pH 1), the amino group is protonated (NH3+) and the carboxyl group is not dissociated (—COOH). As the pH is raised, the carboxylic acid is the first group to give up a proton, because its pKa is near 2. The dipolar form persists until the pH approaches 9, when the protonated amino group loses a proton. Under physiological conditions, amino acids exist in the dipolar form.