3.2 Amino Acids Contain a Wide Array of Functional Groups
Twenty kinds of side chains varying in size, shape, charge, hydrogen-
All proteins in all species—
Although there are many ways to classify amino acids, we will assort these molecules into four groups, on the basis of the general chemical characteristics of their R groups:
Hydrophobic amino acids with nonpolar R groups
Polar amino acids with neutral R groups but the charge is not evenly distributed
Positively charged amino acids with R groups that have a positive charge at physiological pH (pH ≈ 7.4)
Negatively charged amino acids with R groups that have a negative charge at physiological pH
Hydrophobic Amino Acids Have Mainly Hydrocarbon Side Chains
The amino acids having side chains consisting only of hydrogen and carbon are hydrophobic. The simplest amino acid is glycine, which has a single hydrogen atom as its side chain. With two hydrogen atoms bonded to the α-atom, glycine is unique in being achiral. Alanine, the next simplest amino acid, has a methyl group (–CH3) as its side chain (Figure 3.3). The three-
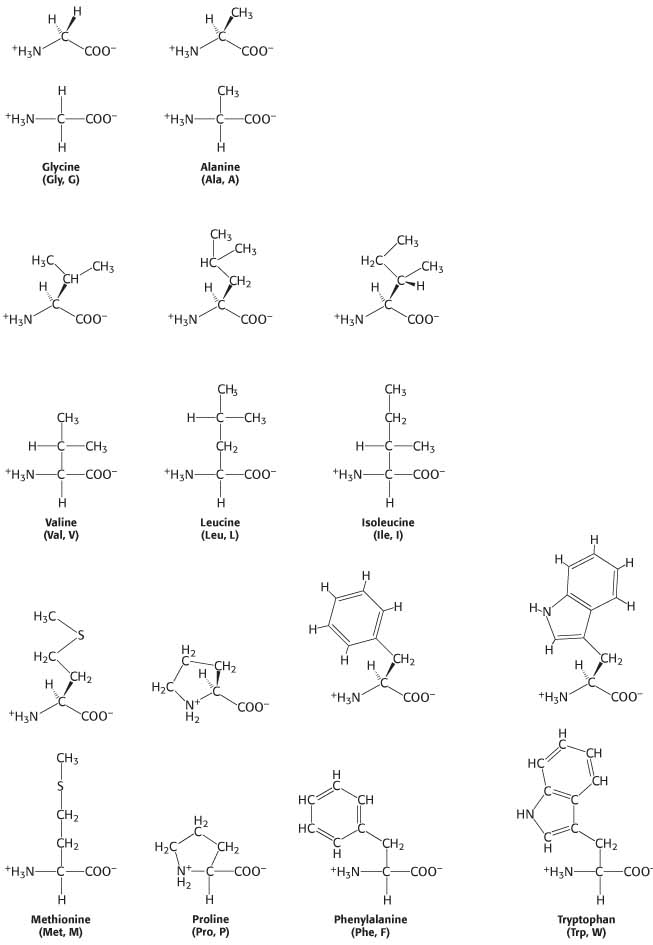
Larger aliphatic side chains are found in the branched-
Two amino acids with simple aromatic side chains are also classified as hydrophobic (Figure 3.3). Phenylalanine, as its name indicates, contains a phenylring attached in place of one of the methyl hydrogen atoms of alanine. Tryptophan has an indole ring joined to a methylene (—CH2—) group; the indole group comprises two fused rings and an NH group.
The hydrophobic amino acids, particularly the larger aliphatic and aromatic ones, tend to cluster together inside the protein away from the aqueous environment of the cell. This tendency of hydrophobic groups to come together is called the hydrophobic effect and is the driving force for the formation of the unique three-
Polar Amino Acids Have Side Chains That Contain an Electronegative Atom
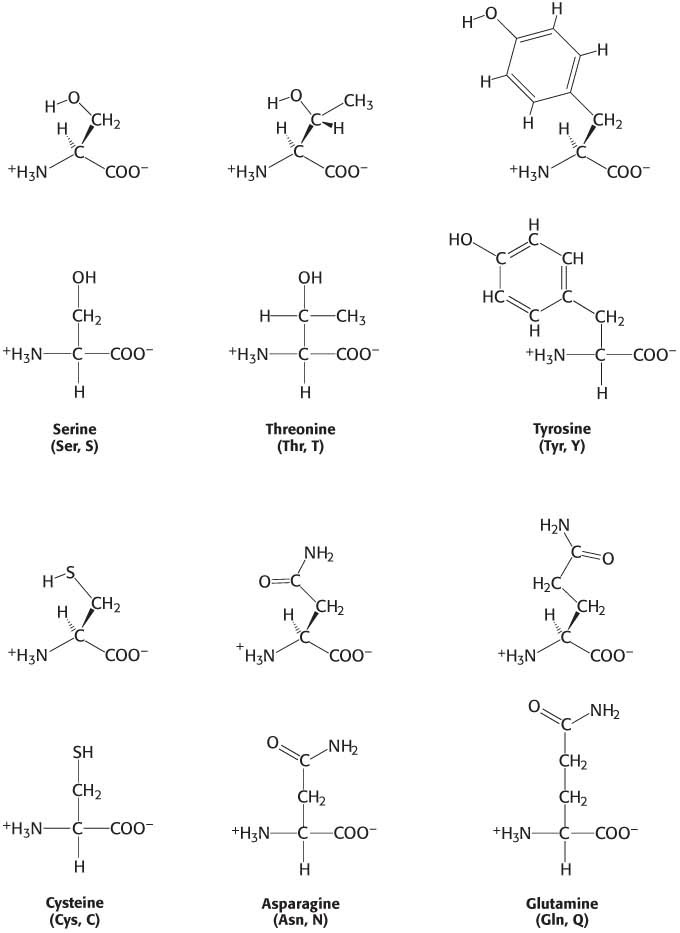
The next group of amino acids that we will consider are those that are neutral overall, yet they are polar because the R group contains an electronegative atom that hoards electrons. Three amino acids, serine, threonine, and tyrosine, contain hydroxyl (—OH) groups (Figure 3.4). The electrons in the O—
DID YOU KNOW?
Lysine is an essential amino acid, which means that human beings cannot synthesize lysine and must obtain it in the diet. In experimental animals, kept on a cereal-
Positively Charged Amino Acids Are Hydrophilic
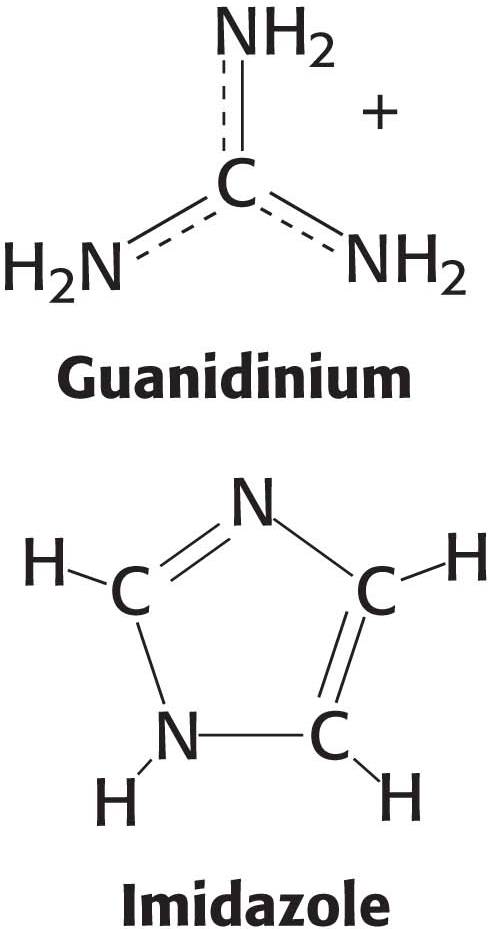
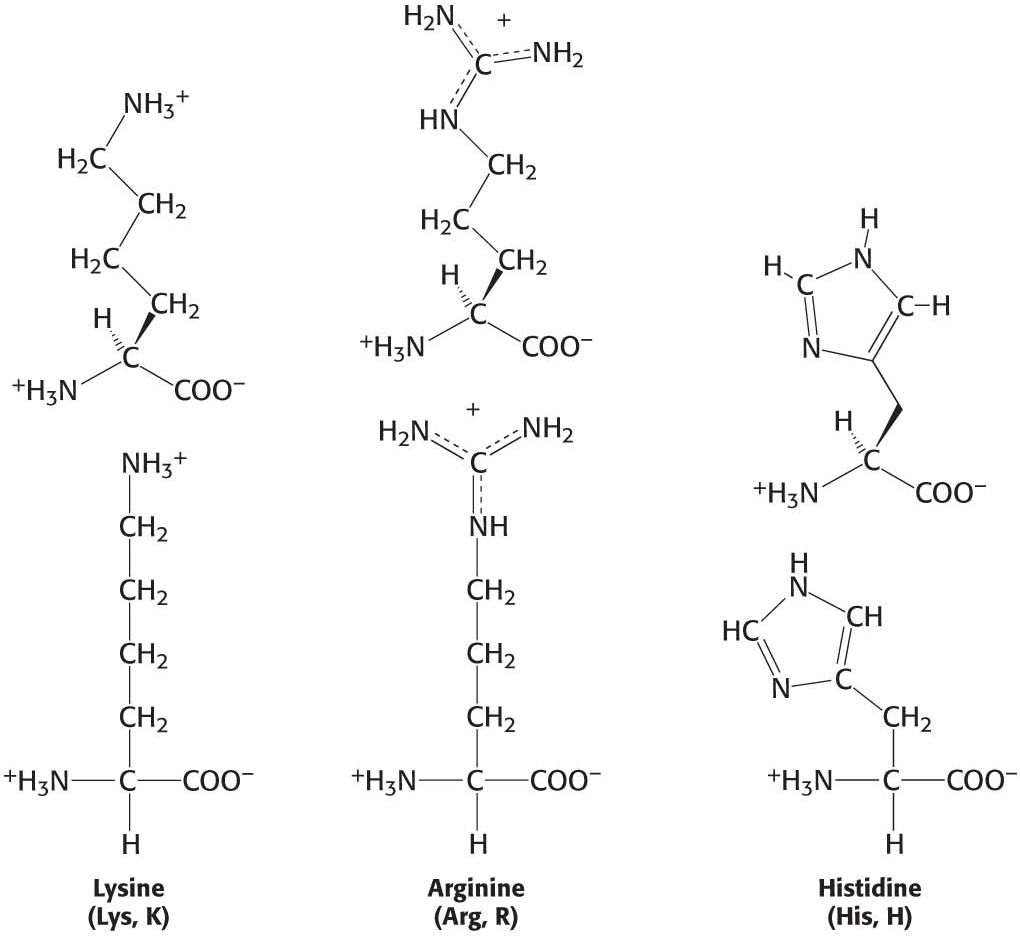
We now turn to amino acids having positively charged side chains that render these amino acids highly hydrophilic (Figure 3.5). Lysine and arginine have long side chains that terminate with groups that are positively charged at neutral pH. Lysine is topped by an amino group and arginine by a guanidinium group. Note that the R groups of lysine and arginine have dual properties—
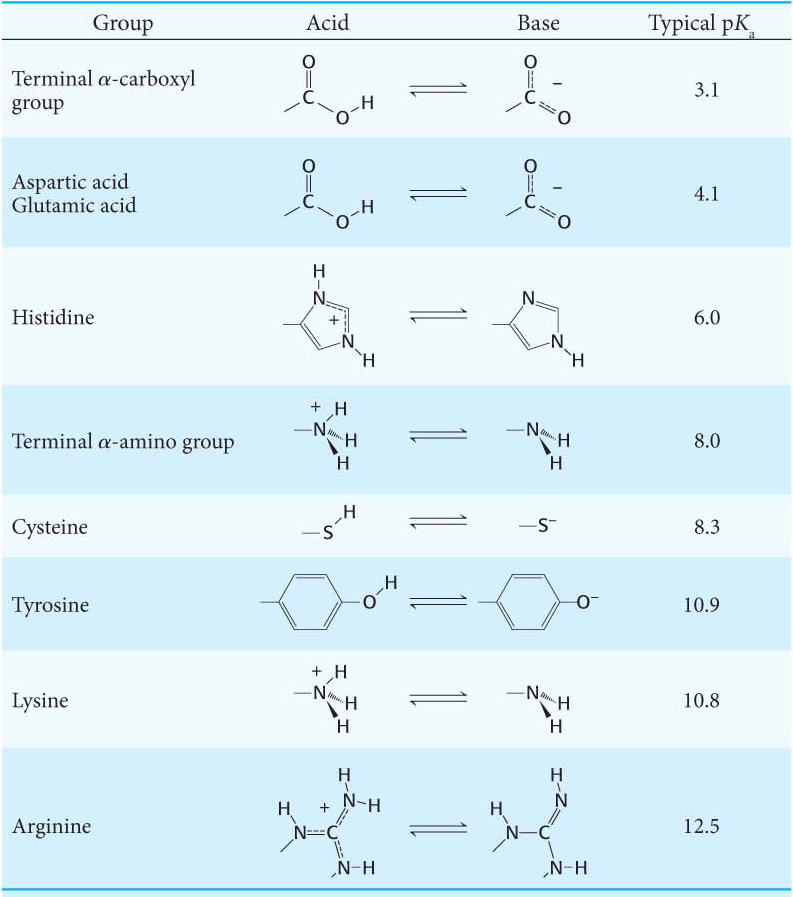
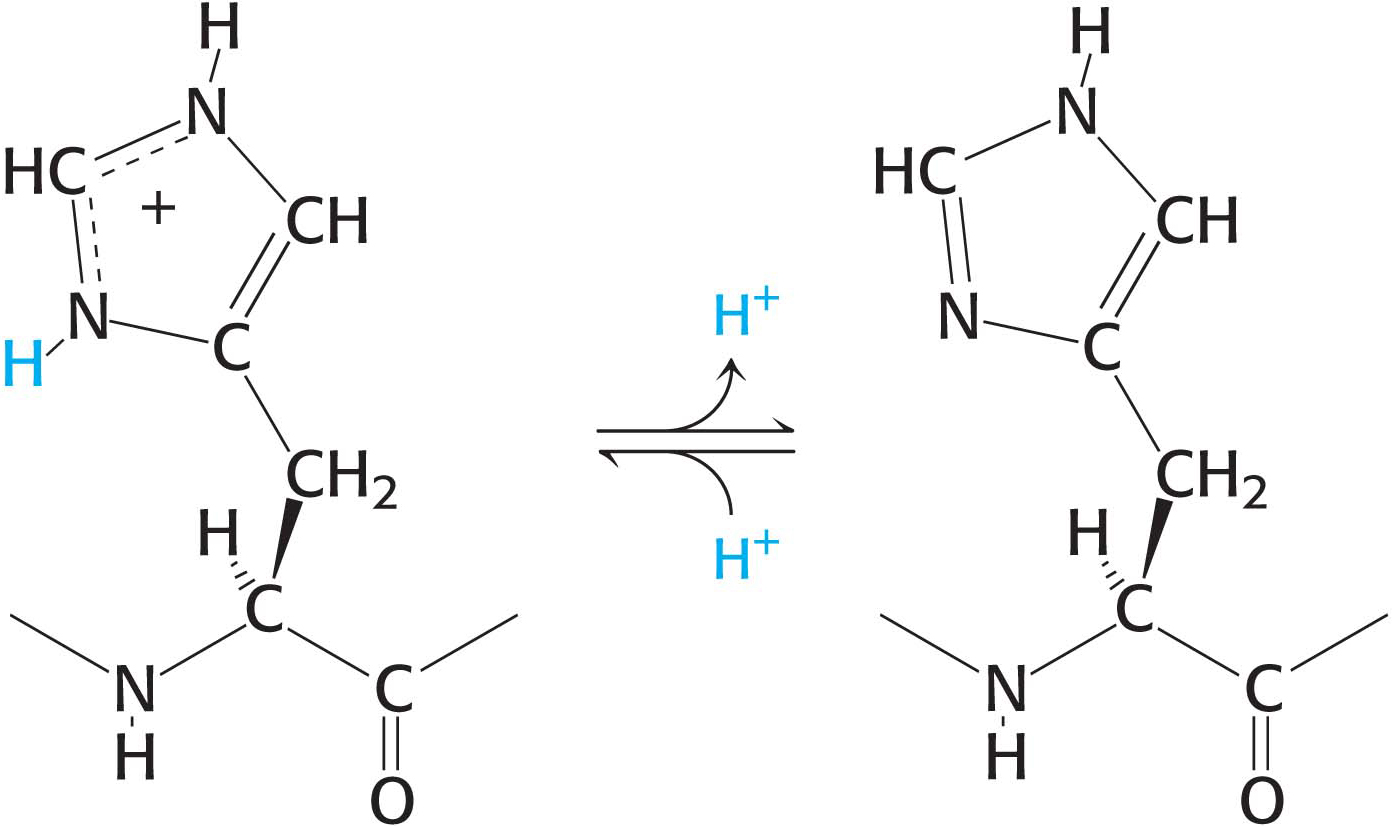
Histidine contains an imidazole group, an aromatic ring that also can be positively charged. With a pKa value near 6, the imidazole group of histidine is unique in that it can be uncharged or positively charged near neutral pH, depending on its local environment (Figure 3.6). Indeed, histidine is often found in the active sites of enzymes, where the imidazole ring can bind and release protons in the course of enzymatic reactions.
Negatively Charged Amino Acids Have Acidic Side Chains
DID YOU KNOW?
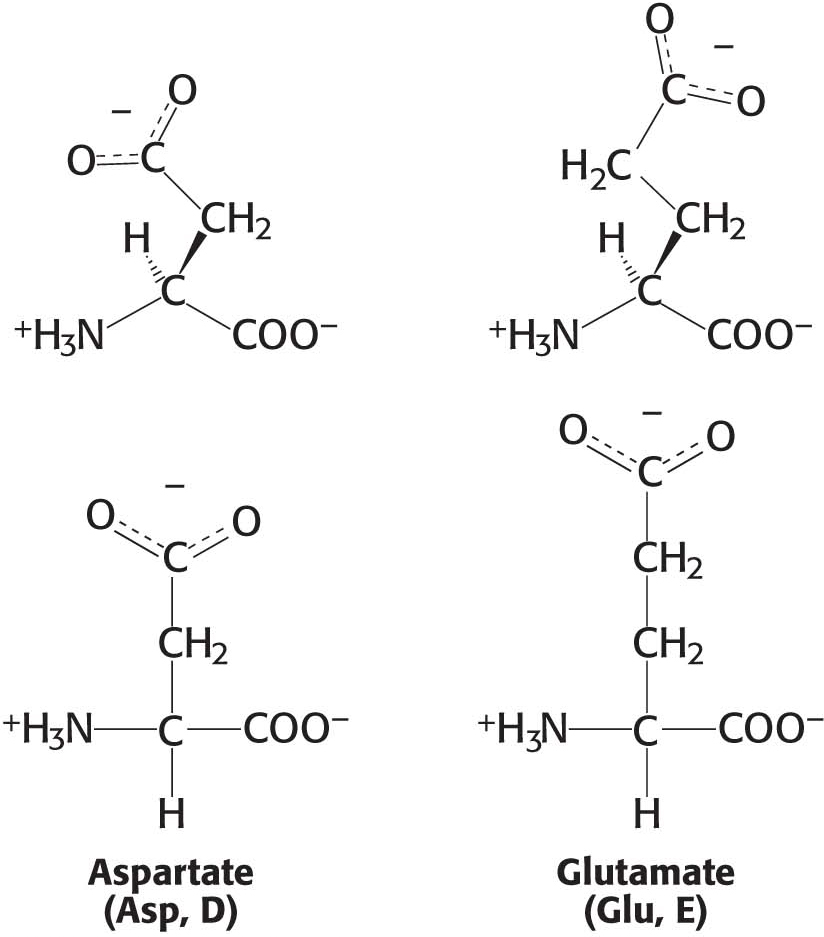
Monosodium glutamate (MSG), which is glutamate with sodium bound to an acid group, is commonly used as a taste enhancer. In fact, the taste of glutamate and aspartate (called umami, from the Japanese word for “deliciousness”) is one of the five primary tastes, the others being sweet, sour, bitter, and salty.
The two amino acids in this group, aspartic acid and glutamic acid, have acidic side chains that are usually negatively charged under intracellular conditions (Figure 3.7). These amino acids are often called aspartate and glutamate to emphasize the presence of the negative charge on their side chains. In some proteins, these side chains accept protons, which neutralize the negative charge. This ability is often functionally important. Aspartate and glutamate are related to asparagine and glutamine in which a carboxylic acid group in the former pair replaces a carboxamide in the latter pair.
The Ionizable Side Chains Enhance Reactivity and Bonding
QUICK QUIZ
Name three amino acids that are positively charged at neutral pH. Name three amino acids that contain hydroxyl groups.
Lysine, arginine, and histidine are positively charged at neutral pH. Three amino acids containing hydroxyl groups are serine, threonine, and tyrosine.
Seven of the 20 amino acids—