PROBLEMS
Question 3.1
1. Where’s Elvis? Translate the following amino acid sequence into one-
Question 3.2
2. A nervous polecat. Pyrrolysine (Pyl, O) and selenocysteine (Sec, U) are two uncommon amino acids. Knowing that these amino acids exist, translate the following amino acid sequence into one-
Question 3.3
3. Identify. Examine the following four amino acids. What are their names, three-
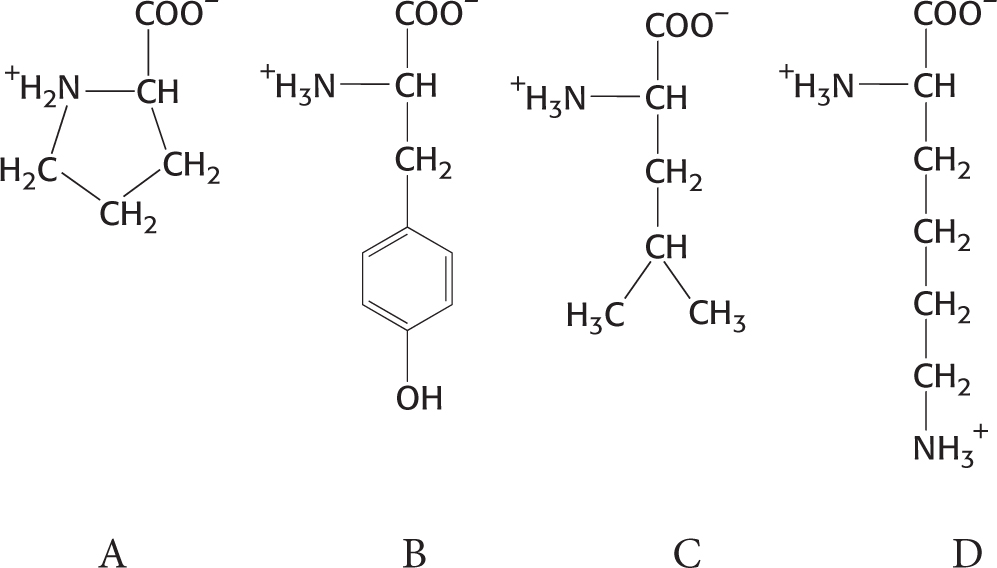
Question 3.4
4. Properties. In reference to the amino acids shown in problem 2, which are associated with the characteristics (a)–(e)? ✓ 1
(a) Hydrophobic side chain_______________
(b) Basic side chain_______________
(c) Three ionizable groups_______________
(d) pKa of approximately 10 in proteins_______________
(e) Modified form of phenylalanine_______________
Question 3.5
5. Match ’em. Match each amino acid in the left-
Leu Glu Lys Ser Cys Trp | acidic hydroxyl- basic nonpolar aromatic sulfur- nonpolar aliphatic |
Question 3.6
6. Solubility. In each of the following pairs of amino acids, identify which amino acid would be most soluble in water:
(a) Ala, Leu;
(b) Tyr, Phe;
(c) Ser, Ala;
(d) Trp, His.
Question 3.7
7. Charge and pH. What is the net charge on the amino acid glycine at pH 7? At pH 12?
Question 3.8
8. Isoelectric point. The isoelectric point (pI) is the pH at which a molecule has no net charge. The amino acid glycine has two ionizable groups: (1) a carboxylic acid group with a pKa of 2.72 and (2) an amino group with a pKa of 9.60. Calculate the pI of glycine.
Question 3.9
9. Positive R. Which amino acids have positively charged R groups at pH 7?
Question 3.10
10. Crucial versus noncrucial. Differentiate between a nonessential and an essential amino acid.
Question 3.11
11. Aromatic, not romantic. What three amino acids have aromatic components in their side chains? ✓ 1
Question 3.12
12. Getting a charge out of it. Which amino acid side chains are capable of ionization? ✓ 1
Question 3.13
13. Different states. An amino acid with two ionizable groups can exist in four possible ionization states. Draw the four possible ionization states for alanine, and indicate which state predominates at pH 1, pH 7, and pH 11. ✓ 1
Question 3.14
14. Carbolic acid-
Question 3.15
15. Bonding is good. Which of the following amino acids have R groups that have hydrogen-
Challenge Problems
Question 3.16
16. Minor species. For an amino acid such as alanine, the major species in solution at pH 7 is the zwitterionic form. Assume a pKa value of 8 for the amino group and a pKa value of 3 for the carboxylic acid. Estimate the ratio of the concentration of the neutral amino acid species (with the carboxylic acid protonated and the amino group neutral) to that of the zwitterionic species at pH 7.
Question 3.17
17. Half-
Question 3.18
18. +/0. The R group of lysine has an amino group that can be positively charged or lose a proton to become neutral. The pKa of the amino group is 10.8. Determine the fraction of amino group that is protonated at pH = 9.8 and at pH = 11.8.
Question 3.19
19. 0/−. Glutamic acid has an R group that contains a carboxyl group whose pKa = 4.1. What fraction of the R group carboxylic acid is protonated at pH = 2.1 and pH = 7.1?
Selected Readings for this chapter can be found online at www.whfreeman.com/