
40.4 A Variety of Biomolecules Can Inhibit Protein Synthesis
Many chemicals that inhibit various aspects of protein synthesis have been identified. These chemicals are powerful experimental tools and clinically useful drugs.
 CLINICAL INSIGHT
CLINICAL INSIGHTSome Antibiotics Inhibit Protein Synthesis
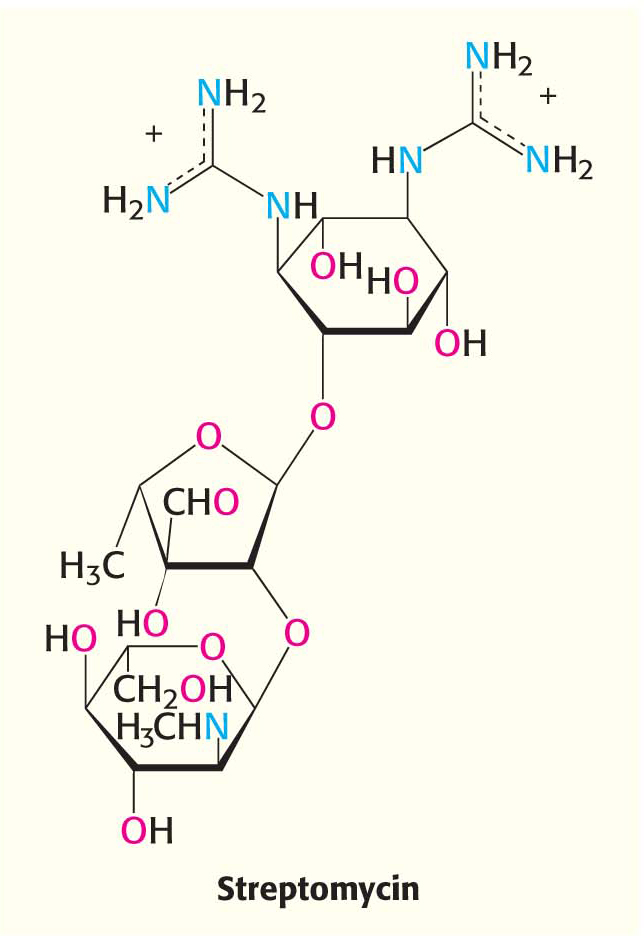
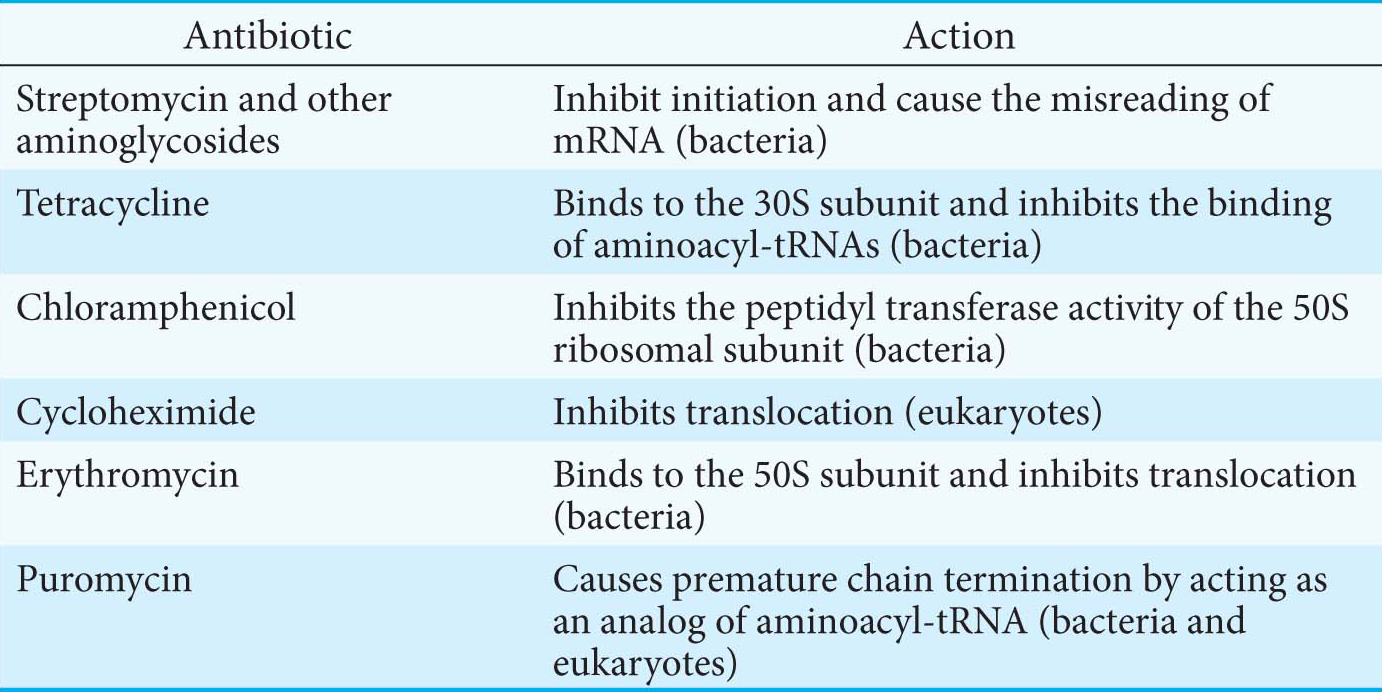
The differences between eukaryotic and bacterial ribosomes can be exploited for the development of antibiotics (Table 40.1). For example, the antibiotic streptomycin, a highly basic trisaccharide, interferes with the binding of formylmethionyl-
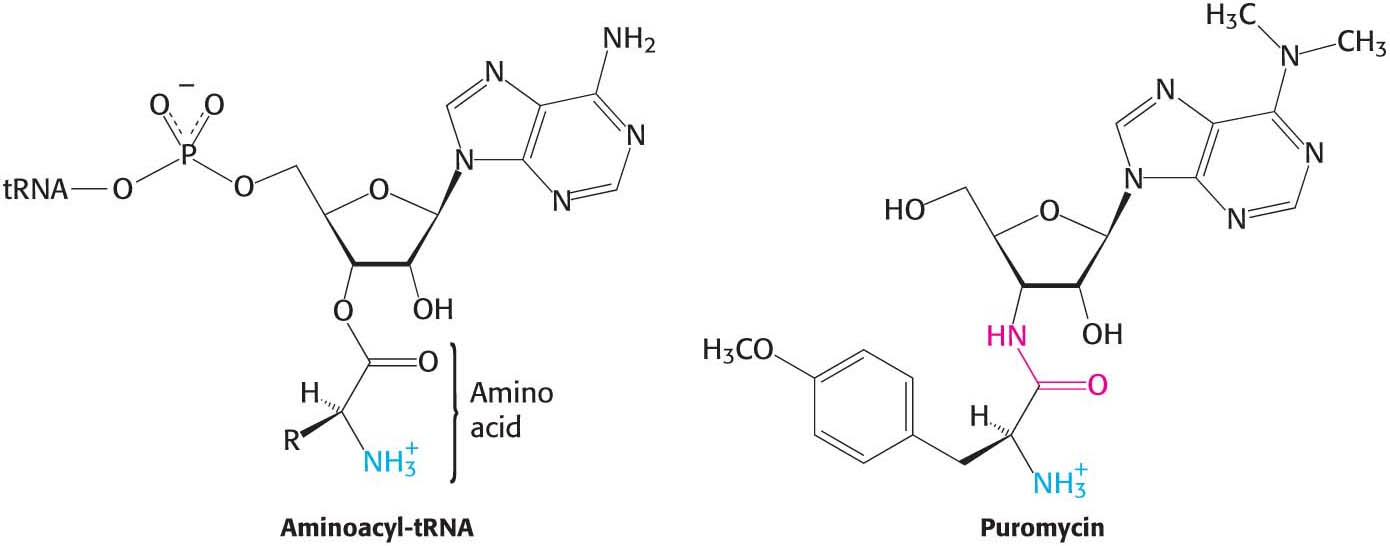
The antibiotic puromycin inhibits protein synthesis in both bacteria and eukaryotes by causing nascent polypeptide chains to be released before their synthesis is completed. Puromycin is an analog of the terminal part of aminoacyl-
DID YOU KNOW?

Pierre Paul Émile Roux (1853–
 CLINICAL INSIGHT
CLINICAL INSIGHTDiphtheria Toxin Blocks Protein Synthesis in Eukaryotes by Inhibiting Translocation
Many antibiotics, harvested from bacteria for medicinal purposes, are inhibitors of bacterial protein synthesis. However, some bacteria produce protein-
A single A fragment of the toxin in the cytoplasm can kill a cell. Why is it so lethal? EF2 contains diphthamide, an unusual amino acid residue that enhances the fidelity of codon shifting during translocation. Diphthamide is formed by a highly conserved complicated pathway that posttranslationally modifies histidine. The A fragment of the diphtheria toxin catalyzes the transfer of the ADP ribose unit of NAD+ to the diphthamide ring (Figure 40.16).
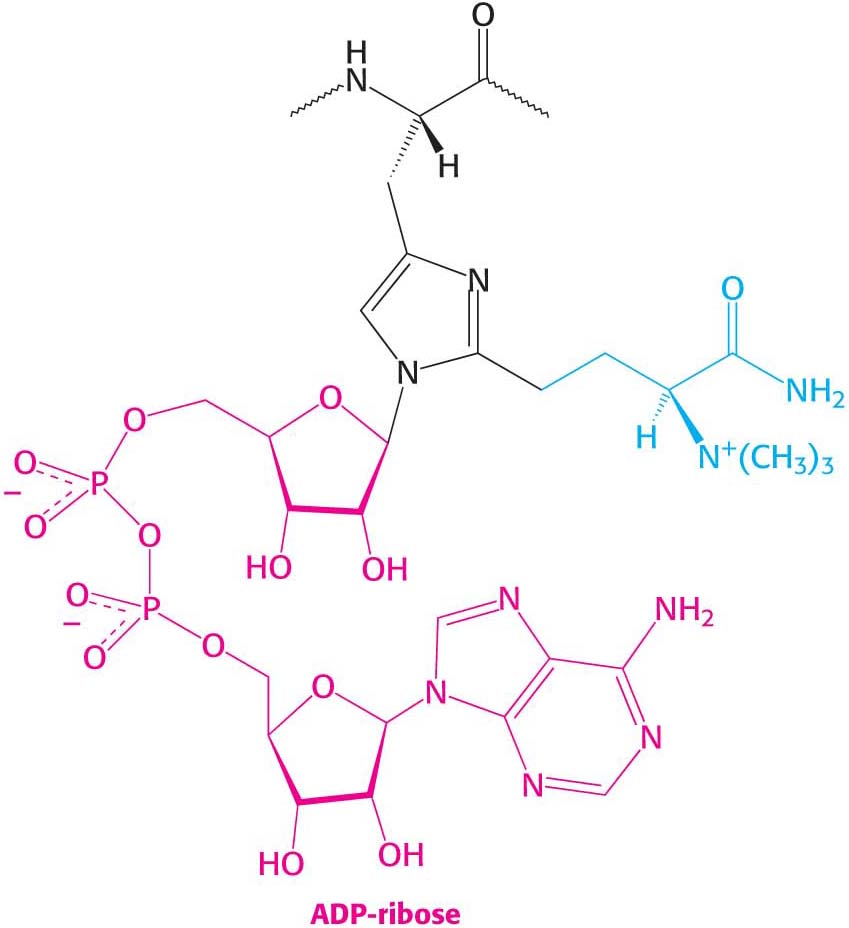
This ADP ribosylation of a single side chain of EF2 blocks EF2’s capacity to carry out the translocation of the growing polypeptide chain. Protein synthesis ceases, accounting for the remarkable toxicity of diphtheria toxin.
DID YOU KNOW?
On September 7, 1978, an agent of the Soviet Union State Security Department (KGB), using a weapon built into an umbrella, embedded a small pellet containing ricin into Bulgarian dissident Georgi Markov’s thigh as he crossed Waterloo Bridge in London. Mr. Markov felt a sharp sting, as if from a bug bite. He died four days later.
 CLINICAL INSIGHT
CLINICAL INSIGHTRicin Fatally Modifies 28S Ribosomal RNA
Ricin is a biomolecule frequently in the news because of its potential use as a bioterrorism agent. Ricin is a small protein (65 kDa) found in the seeds (the beans) of the castor plant, Ricinus communis (Figure 40.17). It is indeed a deadly molecule because as little as 500 mg is lethal for an adult human being and a single molecule can inhibit all protein synthesis in a cell, resulting in cell death.

Ricin is a heterodimeric protein composed of a catalytic A chain joined by a single disulfide bond to a B chain. The B chain allows the toxin to bind to the target cell, and this binding leads to an endocytotic uptake of the dimer and the eventual release of the A chain into the cytoplasm. The A chain is an N-glycosidase that cleaves adenine from a particular adenosine nucleotide on the 28S rRNA that is found in all eukaryotic ribosomes. Removal of the adenine base completely inactivates the ribosome by preventing the binding of elongation factors. Thus, ricin and diphtheria toxin both act by inhibiting protein-