
40.5 Ribosomes Bound to the Endoplasmic Reticulum Manufacture Secretory and Membrane Proteins
Not all newly synthesized proteins are destined to function in the cytoplasm. A newly synthesized protein in E. coli can stay in the cytoplasm or it can be sent to the plasma membrane, the outer membrane, the space between them, or the extracellular medium. Eukaryotic cells can direct proteins to internal sites such as mitochondria, the nucleus, and the endoplasmic reticulum, a process called protein targeting or protein sorting. How is sorting accomplished? There are two general mechanisms by which sorting takes place. In one mechanism, the protein is synthesized in the cytoplasm, and then the completed protein is delivered to its intracellular location posttranslationally. Proteins destined for the nucleus, chloroplast, mitochondria, and peroxisomes are delivered by this general process. The other mechanism, termed the secretory pathway, directs proteins into the endoplasmic reticulum (ER), the extensive membrane system that comprises about half the total membrane of a cell, cotranslationally—
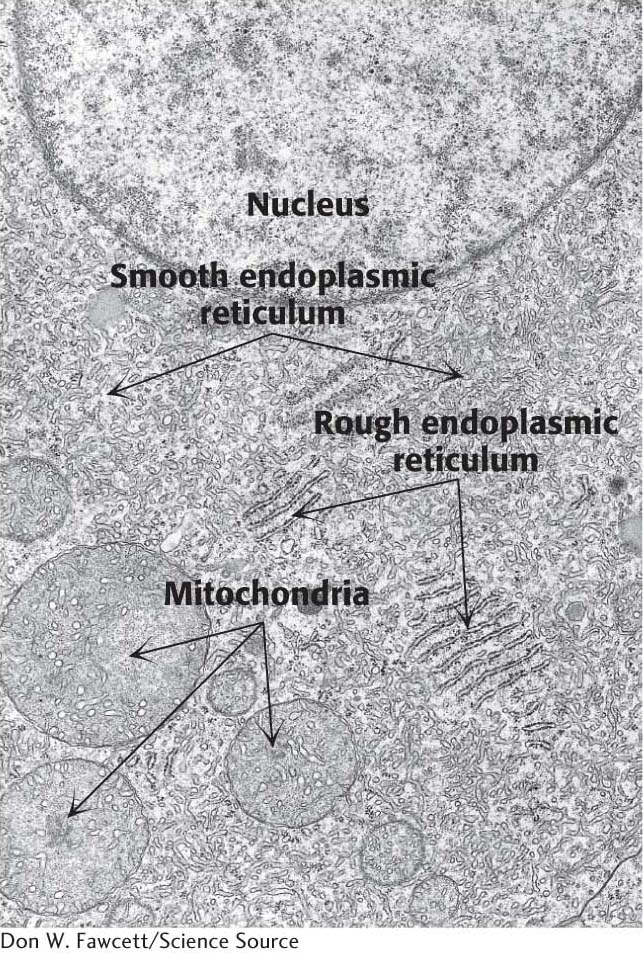
In eukaryotic cells, a ribosome remains free in the cytoplasm unless it is directed to the endoplasmic reticulum. The region that binds ribosomes is called the rough ER because of its studded appearance, as stated in Chapter 1, in contrast with the smooth ER, which is devoid of ribosomes (Figure 40.18).
Protein Synthesis Begins on Ribosomes That Are Free in the Cytoplasm
The synthesis of proteins sorted by the secretory pathway begins on a free ribosome, but shortly after synthesis begins, it is halted until the ribosome is directed to the cytoplasmic side of the endoplasmic reticulum. When the ribosome docks with the membrane, protein synthesis begins again. As the newly forming peptide chain exits the ribosome, it is transported through the membrane into the lumen of the endoplasmic reticulum. Free ribosomes that are synthesizing proteins for use in the cell are identical with those attached to the ER. What is the process that directs the ribosome synthesizing a protein destined to enter the ER to bind to the ER?
Signal Sequences Mark Proteins for Translocation Across the Endoplasmic Reticulum Membrane
The machinery required to direct a ribosome to the ER and to translocate the nascent protein across the ER consists of four components:
The Signal Sequence. The signal sequence is a sequence of 9 to 12 hydrophobic amino acid residues, sometimes containing positively charged amino acids. This sequence, which adopts an α-helical structure, is usually near the amino terminus of the nascent polypeptide chain. The presence of the signal sequence identifies the nascent peptide as one that must cross the ER membrane. Some signal sequences are maintained in the mature protein, whereas others are cleaved by a signal peptidase on the lumenal side of the ER membrane (Figure 40.19).
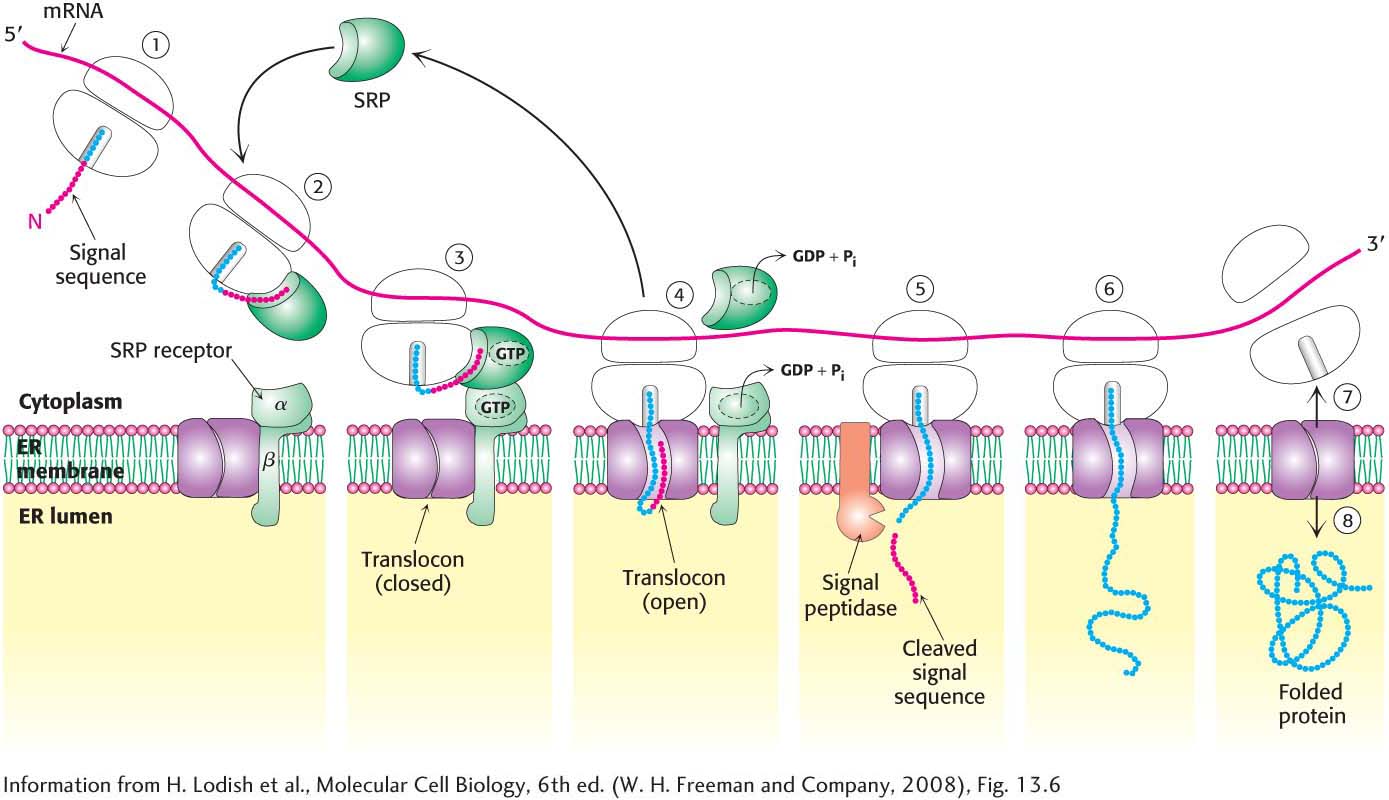 Figure 40.19 The SRP targeting cycle. (1) Protein synthesis begins on free ribosomes. (2) After the signal sequence has exited the ribosome, it is bound by the signal-
Figure 40.19 The SRP targeting cycle. (1) Protein synthesis begins on free ribosomes. (2) After the signal sequence has exited the ribosome, it is bound by the signal-recognition particle (SRP), and protein synthesis halts. (3) The SRP– ribosome complex docks with the SRP receptor in the ER membrane. (4) The ribosome– nascent polypeptide is transferred to the translocon. The SRP and the SRP receptor simultaneously hydrolyze bound GTPs. Protein synthesis resumes, and the SRP is free to bind another signal sequence. (5) The signal peptidase may remove the signal sequence as it enters the lumen of the ER. (6) Protein synthesis continues as the protein is synthesized directly into the ER. (7) On completion of protein synthesis, the ribosome is released and the protein tunnel in the translocon closes. Page 734The Signal-
Recognition Particle . The signal-recognition particle (SRP) is a ribonucleoprotein consisting of a 7S RNA and six different proteins. The SRP recognizes the signal sequence and binds the sequence and the ribosome as soon as the signal sequence exits the ribosome. After the SRP is bound to the signal sequence, interactions between the ribosome and the SRP occlude the EF- binding site, thereby halting protein synthesis. The SRP then shepherds the ribosome and its nascent polypeptide chain to the ER membrane. Like the G proteins considered earlier, the SRP is a GTP- binding protein with GTPase activity. Thus, the SRP samples ribosomes until it locates one exhibiting a signal sequence. The SRP Receptor. The SRP–
ribosome complex travels to the endoplasmic reticulum, where the SRP binds the SRP receptor (SR), an integral membrane protein consisting of two subunits, SRα and SRβ. SRα is, like the SRP, a GTPase. The Translocon. The SRP–
SR complex delivers the ribosome to the translocation machinery, called the translocon, a multisubunit assembly of integral and peripheral membrane proteins. The translocon is a protein- conducting channel . This channel opens when the translocon and ribosome bind to each other. Protein synthesis resumes, with the growing polypeptide chain passing through the translocon channel into the lumen of the ER.
QUICK QUIZ 2
What four components are required for the translocation of proteins across the endoplasmic reticulum membrane?
The signal sequence, the signal-
The interactions of the components of the translocation machinery are shown in Figure 40.19. Both the SRP and the SRα subunit of the SR must bind GTP to facilitate the formation of the SRP-