4.2 Secondary Structure: Polypeptide Chains Can Fold into Regular Structures
Can a polypeptide chain fold into a regularly repeating structure? In 1951, Linus Pauling and Robert Corey proposed that certain polypeptide chains have the ability to fold into two periodic structures called the α helix (alpha helix) and the β pleated sheet (beta pleated sheet). Subsequently, other structures such as turns and loops were identified. Alpha helices, β pleated sheets, and turns are formed by a regular pattern of hydrogen bonds between the peptide NH and CO groups of amino acids that are often near one another in the linear sequence, or primary structure. Such regular folded segments are called secondary structure.
The Alpha Helix Is a Coiled Structure Stabilized by Intrachain Hydrogen Bonds
DID YOU KNOW?
Screw sense refers to the direction in which a helical structure rotates with respect to its axis. If viewed down the axis of a helix (N terminus to C terminus), the chain turns in a clockwise direction; it has a right-
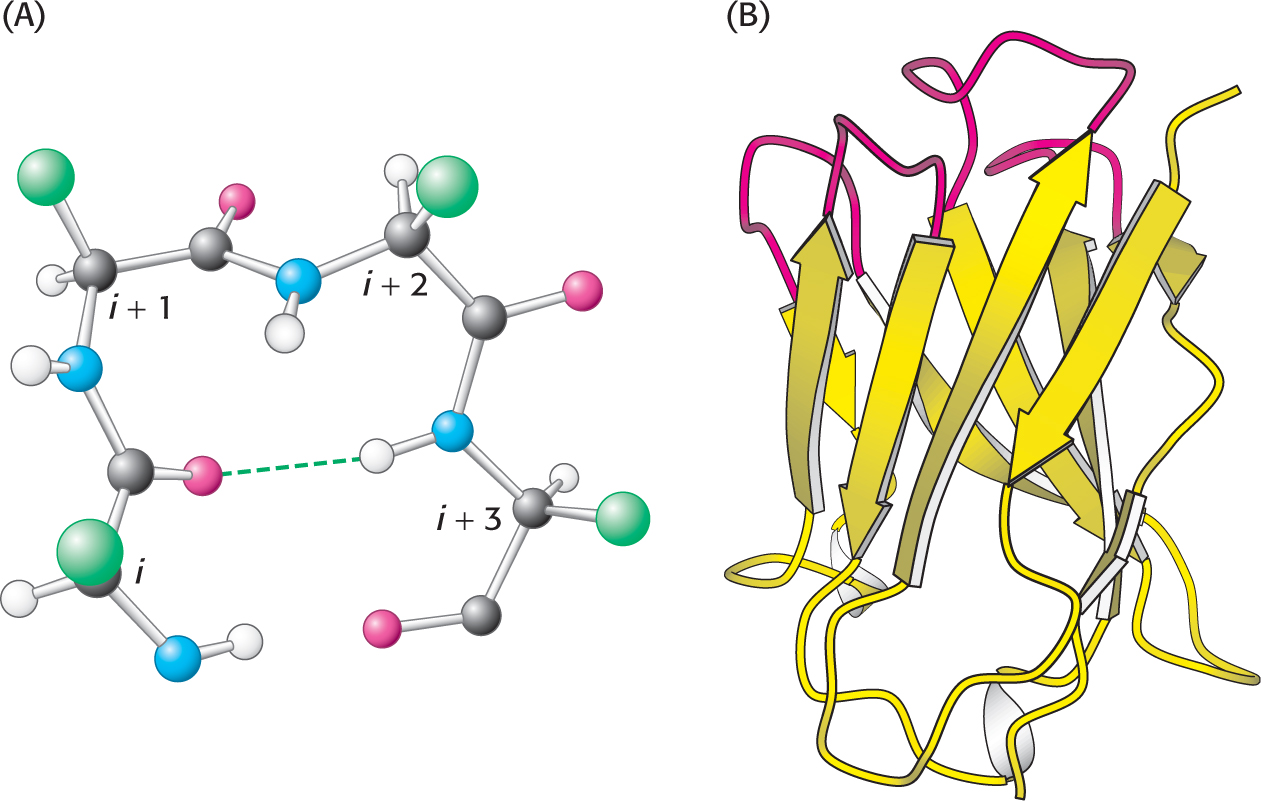
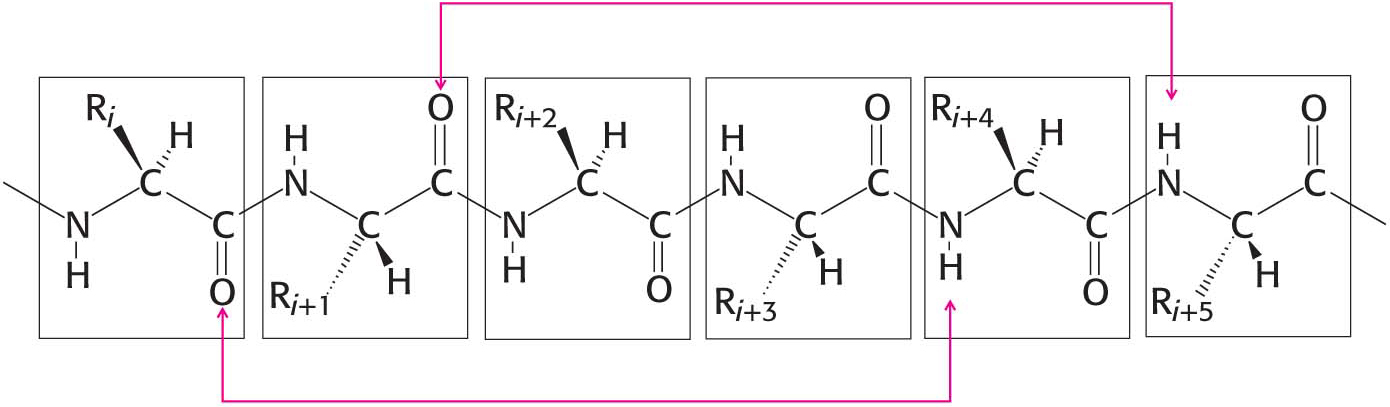
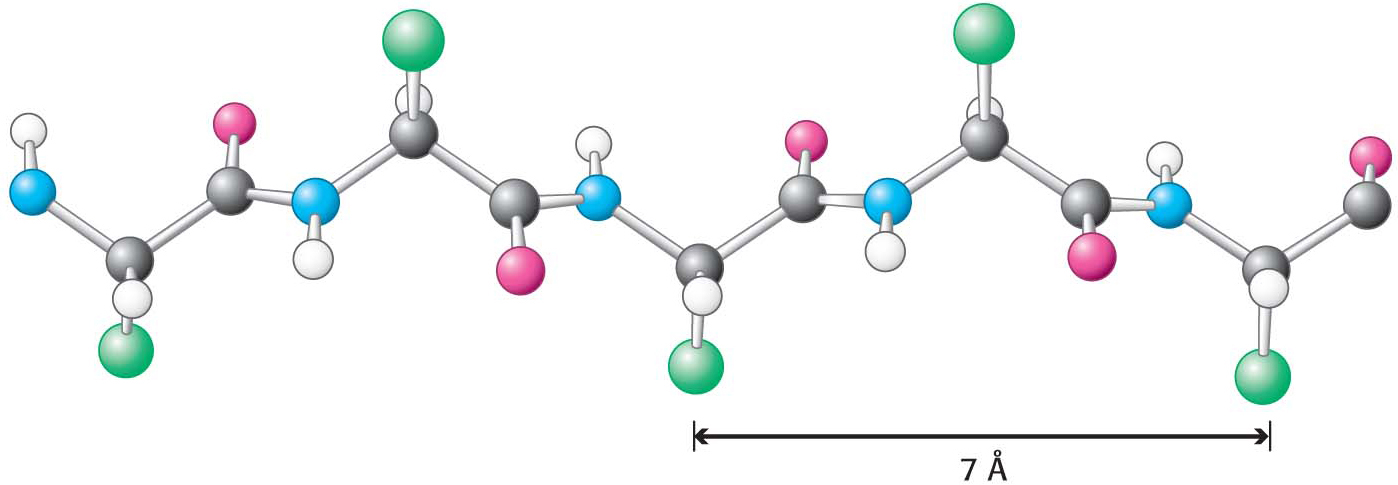
The first of Pauling and Corey’s proposed secondary structures was the α helix, a rodlike structure with a tightly coiled backbone. The side chains of the amino acids composing the structure extend outward in a helical array (Figure 4.11). The α helix is stabilized by hydrogen bonds between the NH and CO groups of the main chain. The CO group of each amino acid forms a hydrogen bond with the NH group of the amino acid that is situated four residues ahead in the sequence (Figure 4.12). Thus, except for amino acids near the ends of an α helix, all the main-
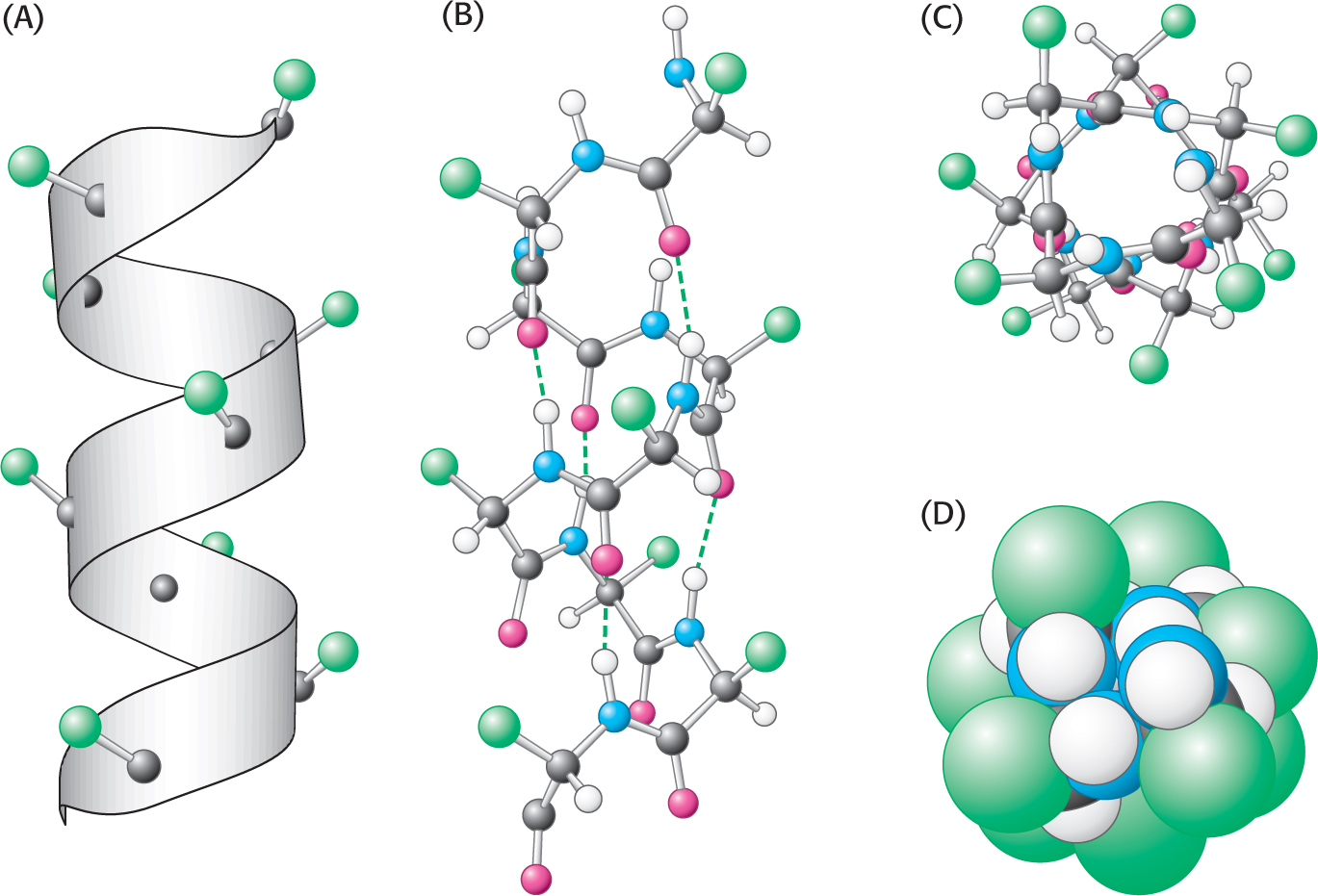
Not all amino acids can be readily accommodated in an α helix. Branching at the β-carbon atom, as in valine, threonine, and isoleucine, tends to destabilize α helices because of steric clashes. Serine, aspartate, and asparagine also tend to disrupt α helices because their side chains contain hydrogen-
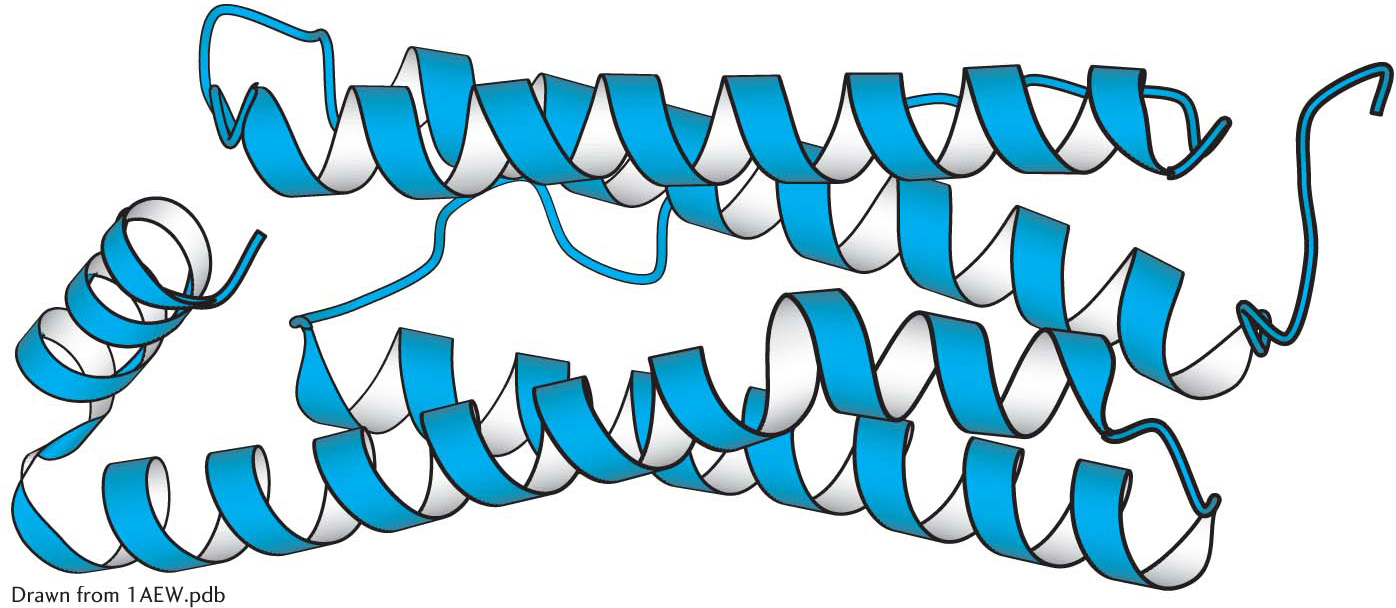
The α-helical content of proteins ranges widely, from none to almost 100%. For example, about 75% of the residues in ferritin, an iron-
Beta Sheets Are Stabilized by Hydrogen Bonding Between Polypeptide Strands
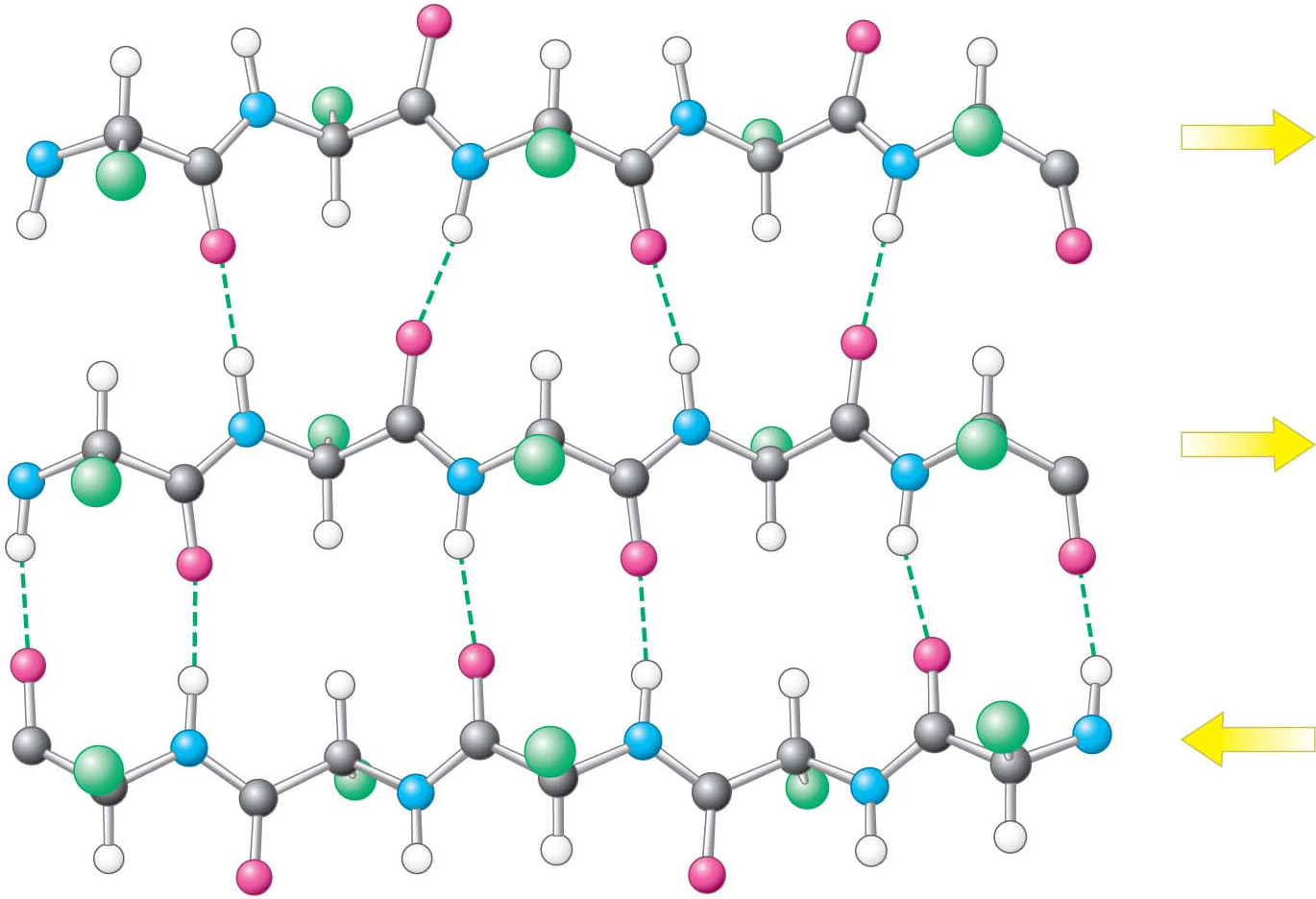
Pauling and Corey named their other proposed periodic structural motif the β pleated sheet (β because it was the second structure that they elucidated). The β pleated sheet (more simply, the β sheet) differs markedly from the rodlike α helix in appearance and bond structure.
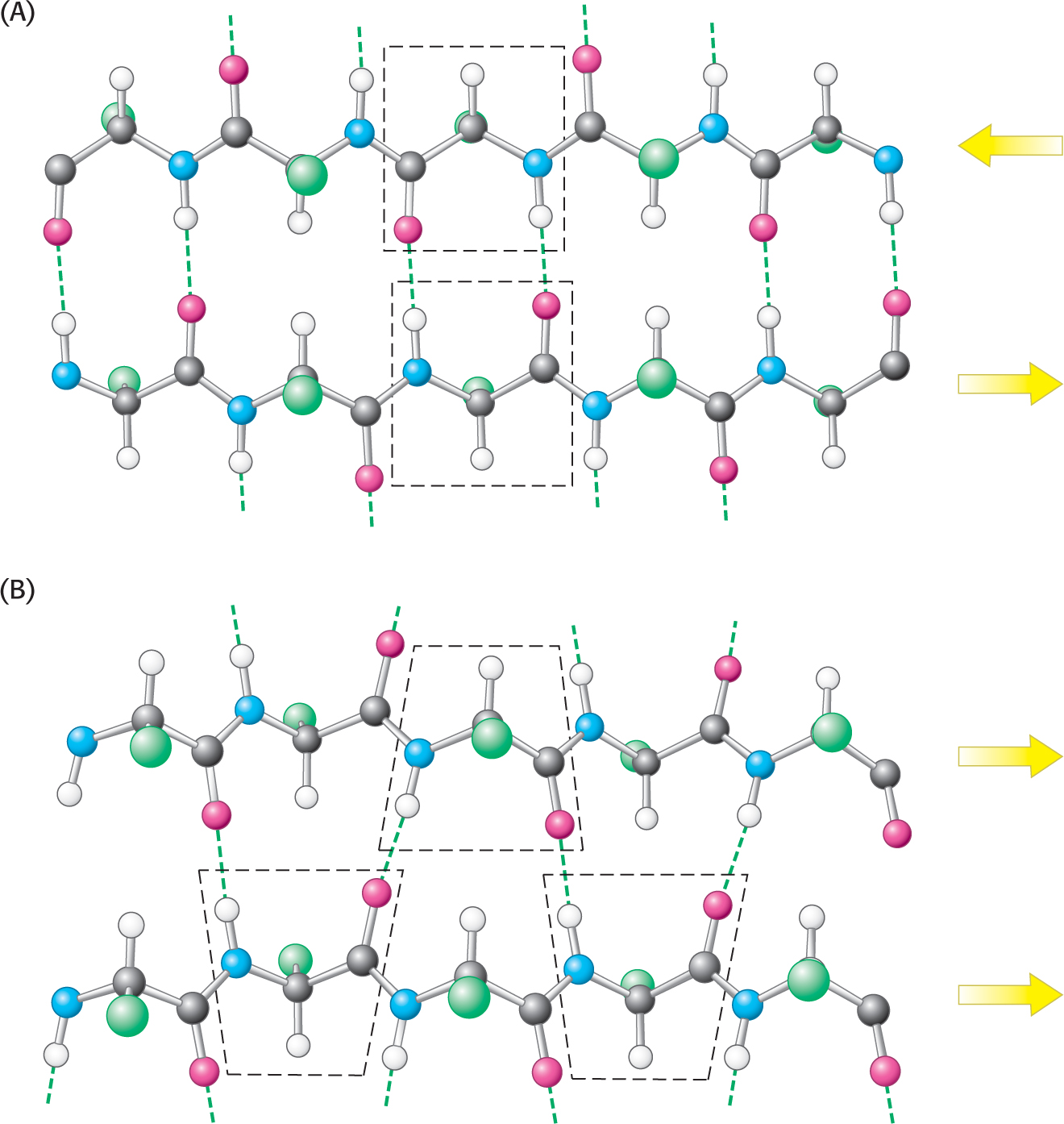
Instead of a single polypeptide strand, the β sheet is composed of two or more polypeptide chains called β strands. A β strand is almost fully extended rather than being tightly coiled as in the α helix. The distance between adjacent amino acids along a β strand is approximately 3.5 Å, in contrast with a distance of 1.5 Å along an α helix. The side chains of adjacent amino acids point in opposite directions (Figure 4.15).
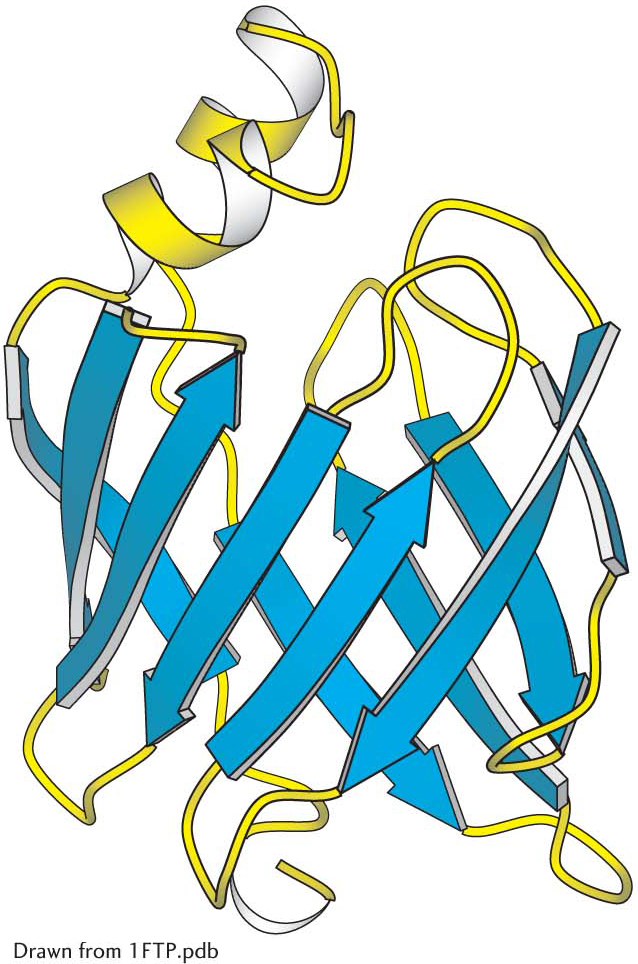
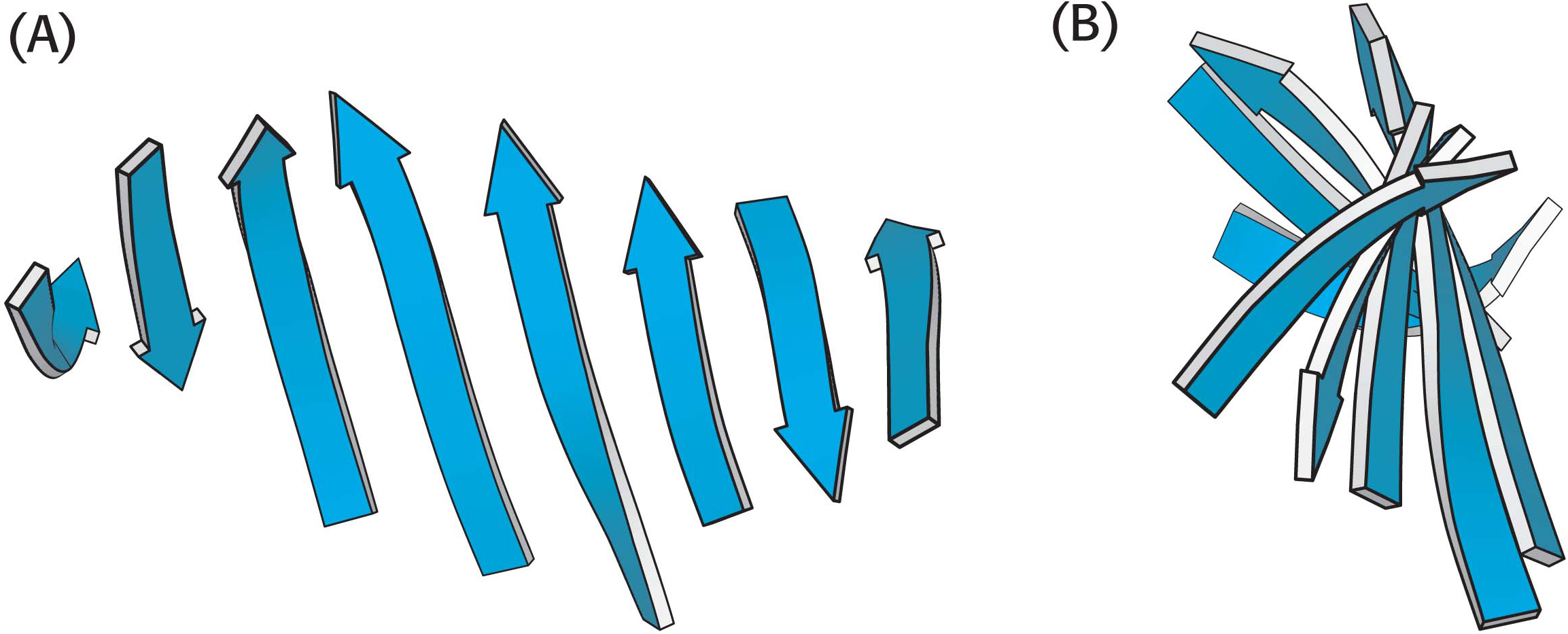
A β sheet is formed by linking two or more β strands lying next to one another through hydrogen bonds. Adjacent chains in a β sheet can run in opposite directions (antiparallel β sheet) or in the same direction (parallel β sheet), as shown in Figure 4.16. Many strands, typically 4 or 5 but as many as 10 or more, can come together in a β sheet. Such β sheets can be purely antiparallel, purely parallel, or mixed (Figure 4.17). Unlike α helices, β sheets can consist of sections of a polypeptide that are not near one another. That is, in two β strands that lie next to each other, the last amino acid of one strand and the first amino acid of the adjacent strand are not necessarily neighbors in the amino acid sequence.
In schematic representations, β strands are usually depicted by broad arrows pointing in the direction of the carboxyl-
Polypeptide Chains Can Change Direction by Making Reverse Turns and Loops
Most proteins have compact globular shapes, requiring reversals in the direction of their polypeptide chains. Many of these reversals are accomplished by common structural elements called reverse turns and loops (Figure 4.20). Turns and loops invariably lie on the surfaces of proteins and thus often participate in interactions between other proteins and the environment. Loops exposed to an aqueous environment are usually composed of amino acids with hydrophilic R groups.
Fibrous Proteins Provide Structural Support for Cells and Tissues
Fibrous proteins form long fibers that serve a structural role. Although some of these proteins have regions of complex three-
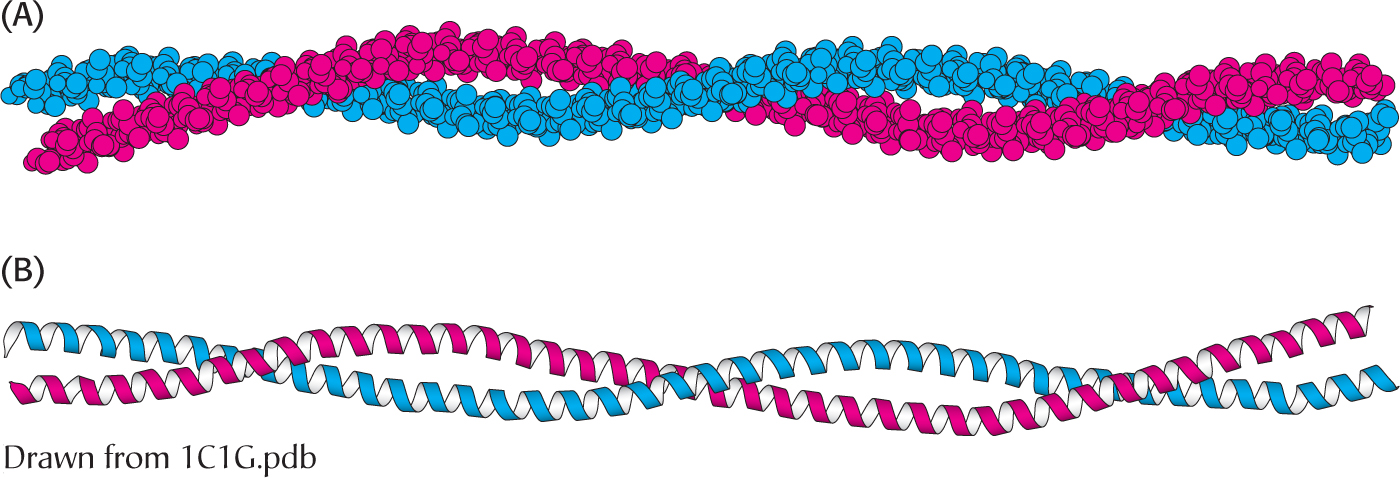

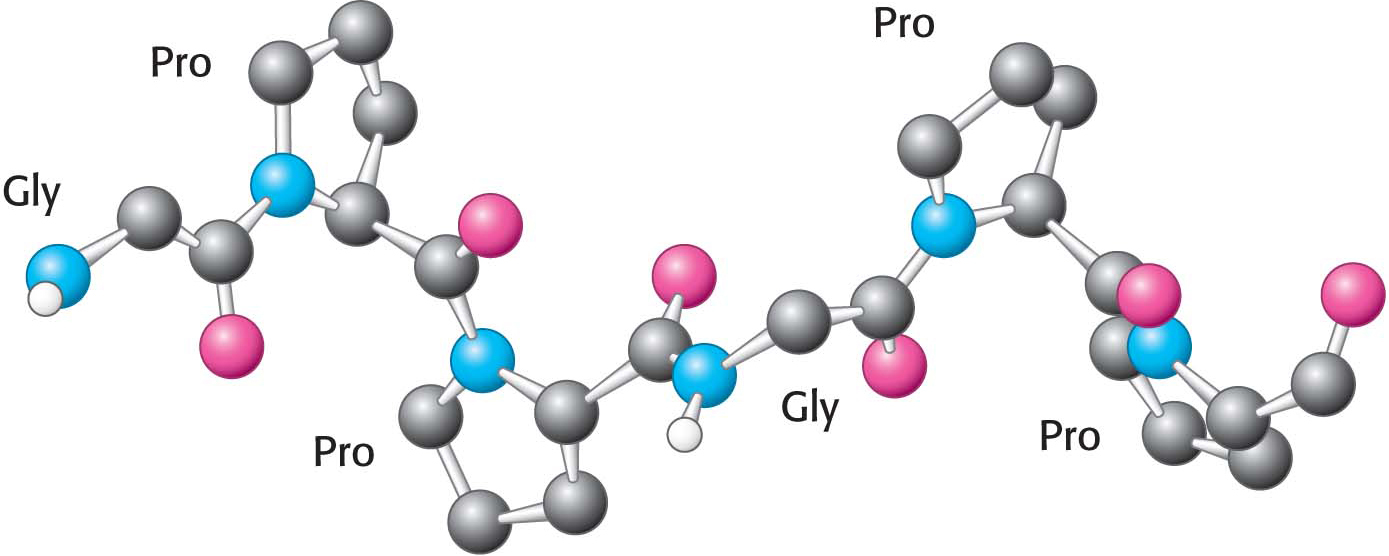
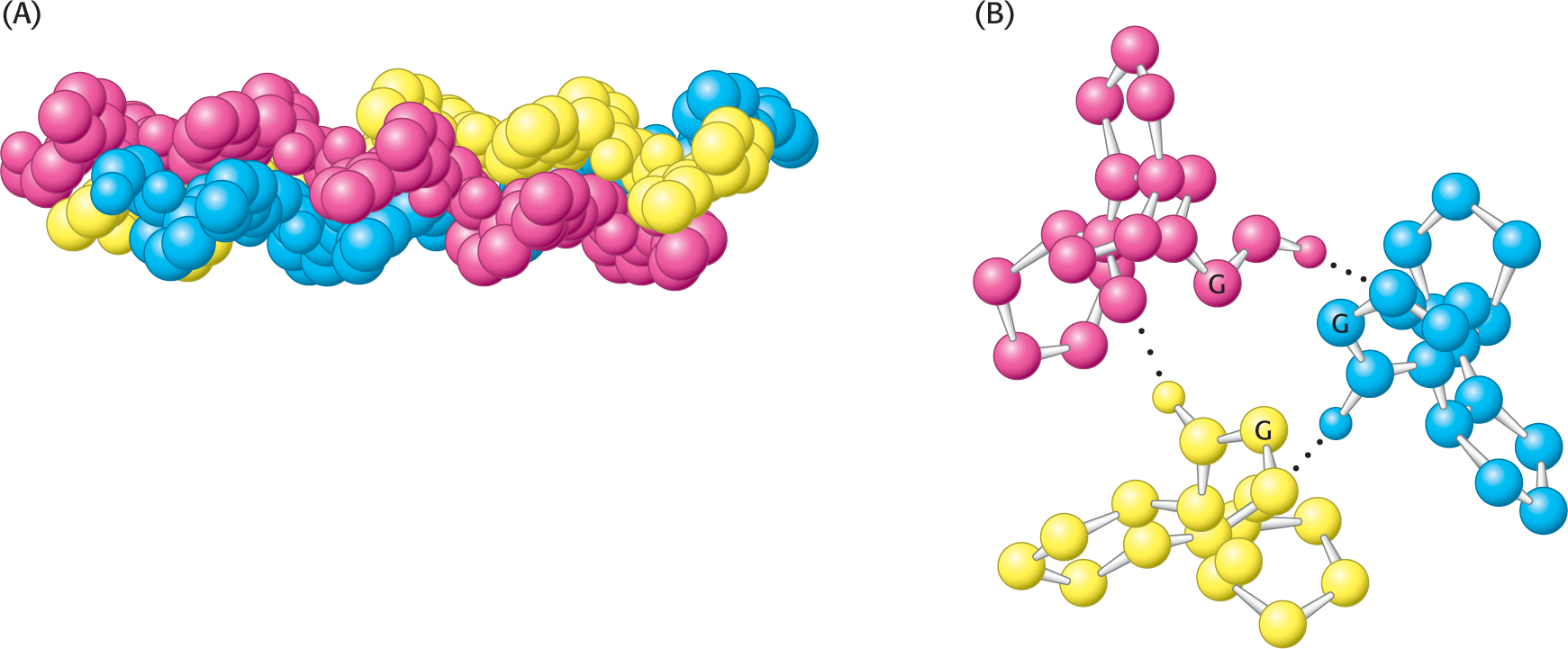
A different type of helix is present in collagen, the most abundant mammalian protein. Collagen is the main fibrous component of skin, bone, tendon, cartilage, and teeth. It contains three helical polypeptide chains, each nearly 1000 residues long. Glycine appears at every third residue in the amino acid sequence, and the sequence glycine-
Hydrogen bonds within each peptide chain are absent in this type of helix. Instead, the helices are stabilized by steric repulsion of the pyrrolidine rings of the proline residues (Figure 4.23). The pyrrolidine rings keep out of each other’s way when the polypeptide chain assumes its helical form, which has about three residues per turn. Three strands wind around each other to form a superhelical cable that is stabilized by hydrogen bonds between strands. The hydrogen bonds form between the peptide NH groups of glycine residues and the CO groups of residues on the other chains. The inside of the triple-
NUTRITION FACTS: Vitamin C

 CLINICAL INSIGHT
CLINICAL INSIGHTDefects in Collagen Structure Result in Pathological Conditions
The importance of the positioning of glycine inside the triple helix is illustrated in the disorder osteogenesis imperfecta, also known as brittle bone disease. In this condition, which can vary from mild to very severe, other amino acids replace the internal glycine residue. This replacement leads to a delayed and improper folding of collagen, and the accumulation of defective collagen results in cell death. The most serious symptom is severe bone fragility. Defective collagen in the eyes causes the whites of the eyes to have a blue tint (blue sclera).
As we have seen, proline residues are important in creating the superhelical cable structure of collagen. Hydroxyproline is a modified version of proline, with a hydroxyl group replacing a hydrogen atom in the pyrrolidine ring. It is a common element of collagen, appearing in the glycine-
Vitamin C is required for the formation of stable collagen fibers because it assists in the formation of hydroxyproline from proline. Less stable collagen can result in scurvy. The symptoms of scurvy include skin lesions and blood-
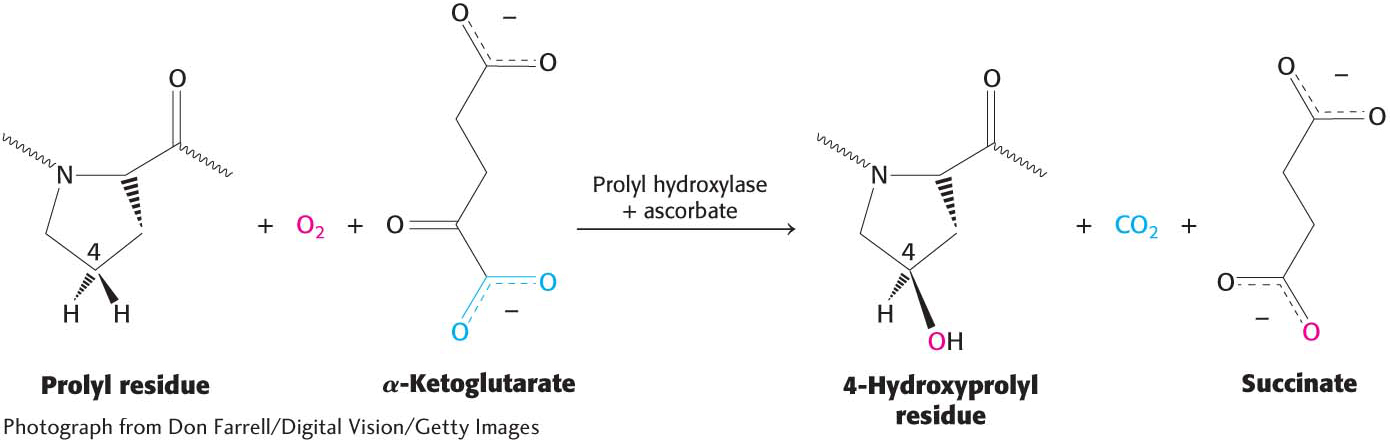
This reaction requires an Fe2+ ion to activate O2. This iron ion, embedded in prolyl hydroxylase, is susceptible to oxidation, which inactivates the enzyme. How is the enzyme made active again? Ascorbate (vitamin C) comes to the rescue by reducing the Fe3+ of the inactivated enzyme. Thus, ascorbate serves here as a specific antioxidant.